当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Noncompetitive Type Three Secretion System ATPase Inhibitors Shut Down Shigella Effector Secretion.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00431 Heather B Case 1 , Dominic S Mattock 2 , Bill R Miller 2 , Nicholas E Dickenson 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00431 Heather B Case 1 , Dominic S Mattock 2 , Bill R Miller 2 , Nicholas E Dickenson 1
Affiliation
|
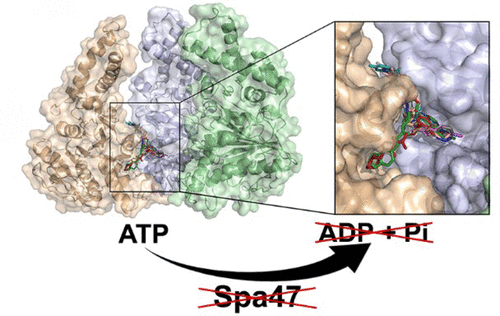
Shigella is the causative agent of bacillary dysentery and is responsible for an estimated 165 million infections and 600,000 deaths annually. Like many Gram-negative pathogens, Shigella relies on a type three secretion system (T3SS) to initiate and sustain infection by directly injecting effector proteins into host cells. Protein secretion through the needle-like injectisome and overall Shigella virulence rely on the T3SS ATPase Spa47, making it a likely means for T3SS regulation and an attractive target for therapeutic small molecule inhibitors. Here, we utilize a recently solved 2.15 Å crystal structure of Spa47 to computationally screen 7.6 million drug-like compounds for candidates which avoid the highly conserved active site by targeting a distal, but critical, interface between adjacent protomers of the Spa47 homohexamer. Ten of the top inhibitor candidates were characterized, identifying novel Spa47 inhibitors that reduce in vitro ATPase activity by as much as 87.9 ± 10.5% with IC50’s as low as 25 ± 20 μM and reduce in vivo Shigella T3SS protein secretion by as much as 94.7 ± 3.0%. Kinetic analyses show that the inhibitors operate through a noncompetitive mechanism that likely supports the inhibitors’ low cytotoxicity, as they avoid off-target ATPases involved in either Shigella or mammalian cell metabolism. Interestingly, the inhibitors display nearly identical inhibition profiles for Spa47 and the T3SS ATPases EscN from E. coli and FliI from Salmonella. Together, the results of this study provide much-needed insight into T3SS ATPase inhibition mechanisms and a strong platform for developing broadly effective cross-pathogen T3SS ATPase inhibitors.
中文翻译:

新型非竞争性三型分泌系统ATPase抑制剂可关闭志贺氏菌效应物的分泌。
志贺氏菌是细菌性痢疾的病原体,估计每年导致1.65亿例感染和60万例死亡。像许多革兰氏阴性病原体一样,志贺氏菌依靠三型分泌系统(T3SS)通过将效应子蛋白直接注入宿主细胞来引发和维持感染。通过针状注射体和整体志贺氏菌分泌蛋白质毒力依赖于T3SS ATPase Spa47,使其成为T3SS调控的可能手段,并成为治疗性小分子抑制剂的诱人靶标。在这里,我们利用了最近解决的Spa47的2.15Å晶体结构,通过靶向Spa47同六聚体的相邻protomer之间的远端但至关重要的界面,筛选了760万个药物样化合物,从而避开了高度保守的活性位点。鉴定了十种顶级抑制剂,鉴定出了新型Spa47抑制剂,它们可将体外ATPase活性降低多达87.9±10.5%,而IC 50则低至25±20μM,并可减少体内志贺氏菌T3SS蛋白分泌高达94.7±3.0%。动力学分析表明,该抑制剂通过一种非竞争性机制起作用,该机制很可能支持该抑制剂的低细胞毒性,因为它们避免了志贺氏菌或哺乳动物细胞代谢中涉及的脱靶ATPase 。有趣的是,这些抑制剂对Spa47和大肠杆菌的T3SS ATPases EscN和沙门氏菌的FliI表现出几乎相同的抑制作用。总之,这项研究的结果为T3SS ATPase抑制机制提供了急需的见解,并为开发广泛有效的交叉病原体T3SS ATPase抑制剂提供了强大的平台。
更新日期:2020-07-21
中文翻译:

新型非竞争性三型分泌系统ATPase抑制剂可关闭志贺氏菌效应物的分泌。
志贺氏菌是细菌性痢疾的病原体,估计每年导致1.65亿例感染和60万例死亡。像许多革兰氏阴性病原体一样,志贺氏菌依靠三型分泌系统(T3SS)通过将效应子蛋白直接注入宿主细胞来引发和维持感染。通过针状注射体和整体志贺氏菌分泌蛋白质毒力依赖于T3SS ATPase Spa47,使其成为T3SS调控的可能手段,并成为治疗性小分子抑制剂的诱人靶标。在这里,我们利用了最近解决的Spa47的2.15Å晶体结构,通过靶向Spa47同六聚体的相邻protomer之间的远端但至关重要的界面,筛选了760万个药物样化合物,从而避开了高度保守的活性位点。鉴定了十种顶级抑制剂,鉴定出了新型Spa47抑制剂,它们可将体外ATPase活性降低多达87.9±10.5%,而IC 50则低至25±20μM,并可减少体内志贺氏菌T3SS蛋白分泌高达94.7±3.0%。动力学分析表明,该抑制剂通过一种非竞争性机制起作用,该机制很可能支持该抑制剂的低细胞毒性,因为它们避免了志贺氏菌或哺乳动物细胞代谢中涉及的脱靶ATPase 。有趣的是,这些抑制剂对Spa47和大肠杆菌的T3SS ATPases EscN和沙门氏菌的FliI表现出几乎相同的抑制作用。总之,这项研究的结果为T3SS ATPase抑制机制提供了急需的见解,并为开发广泛有效的交叉病原体T3SS ATPase抑制剂提供了强大的平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号