当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noble Gases in Carbonate Melts: Constraints on the Solubility and the Surface Tension by Molecular Dynamics Simulation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acsearthspacechem.0c00020 Elsa Desmaele 1 , Nicolas Sator 1 , Bertrand Guillot 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acsearthspacechem.0c00020 Elsa Desmaele 1 , Nicolas Sator 1 , Bertrand Guillot 1
Affiliation
|
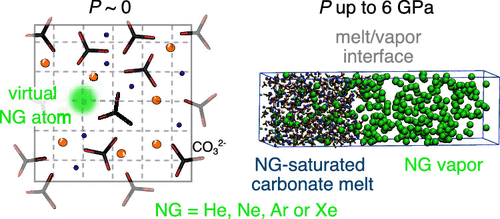
Although they are rare elements in the earth’s mantle, noble gases (NGs) are important geochemical tracers because of their chemical inertness, the specificity of their isotopes, and their strongly varying sizes (a factor >50 in mass from He to Rn). When partial melting occurs at depth, the partitioning of NGs between phases is controlled by a distribution coefficient that can be determined from the solubility of the NGs in each phase. Here, we report quantitative calculations of the solubility of He, Ne, Ar, and Xe in carbonate melts based on molecular dynamics simulations. The NG solubilities are first calculated in K2CO3–CaCO3 mixtures at 1 bar and favorably compared to the only experimental data available to date. Then, we investigate the effect of pressure (up to 6 GPa), focusing on two melt compositions: a dolomitic one and a natrocarbonatitic one (modeling the lava emitted at Ol Doinyo Lengai, Tanzania). The solubility decreases with the amount of alkaline earth cation in the melt and with the size of the noble gas. In the natrocarbonatitic melt, Henry’s law is fulfilled at low pressures (up to ∼0.1 GPa). At higher pressures (a few GPa), the solubility levels off or even starts to diminish smoothly (for He at P > 2 GPa and Ar at P > 4 GPa). In contrast, in molten dolomite, the effect of pressure is negligible on the studied P range (3–6 GPa). At the pressures in the upper part of the upper mantle, the solubilities of NGs in carbonate melts are still of the same order of magnitude as the ones in molten silicates (100 to 101 mol %). This suggests that carbonatitic melts at depth are not preferential carriers of NGs, even if the dependence with the melt composition is not negligible and has to be evaluated on a case-by-case basis. Finally, we evaluate the surface tension at the interface between carbonate melts and NGs and its evolution with pressure. Whatever the composition of the melt and of the NG phase is, the surface tension increases (by a factor ∼2) when P increases from 0 to 6 GPa. This behavior is in contrast to the situation occurring when H2O is in contact with silicate melts (then surface tension drops when pressure increases to a few GPa).
中文翻译:

碳酸盐熔体中的稀有气体:通过分子动力学模拟限制溶解度和表面张力
尽管稀有气体(NGs)是地球地幔中的稀有元素,但由于它们的化学惰性,同位素的特异性以及大小变化很大(从He到Rn的质量系数> 50),它们是重要的地球化学示踪剂。当在深度发生部分熔化时,各相之间NG的分配受分布系数控制,该分布系数可由各相中NG的溶解度确定。在这里,我们报告了基于分子动力学模拟的He,Ne,Ar和Xe在碳酸盐熔体中溶解度的定量计算。NG溶解度首先在K 2 CO 3 –CaCO 3中计算混合物在1 bar的压力下与迄今为止唯一可用的实验数据进行了比较。然后,我们研究压力(高达6 GPa)的影响,重点关注两种熔体成分:白云岩和纳碳碳酸盐(模拟坦桑尼亚Ol Doinyo Lengai排放的熔岩)。溶解度随着熔体中碱土金属阳离子的量以及稀有气体的大小而降低。在碳纳米碳熔体中,亨利定律在低压(最高约0.1 GPa)下满足。在较高的压力(几GPa)下,溶解度趋于平稳甚至开始下降(对于He在P > 2 GPa和Ar在P > 4 GPa时)。相反,在熔融白云岩中,压力对所研究的P的影响可忽略不计范围(3–6 GPa)。在上地幔上部的压力下,碳酸盐熔体中NG的溶解度仍与熔融硅酸盐中的NG溶解度相同(10 0至10 1 mol%)。这表明,即使无法忽略与深度成分的依赖关系,并且必须根据具体情况进行评估,深度的碳酸盐熔体也不是NG的优先携带者。最后,我们评估了碳酸盐熔体与天然气之间的界面处的表面张力及其在压力作用下的演化。无论熔体和NG相的成分是什么,当P从0 GPa增加到6 GPa时,表面张力都会增加(约2倍)。此行为与H 2发生时的情况相反O与硅酸盐熔体接触(然后,当压力增加到几GPa时,表面张力下降)。
更新日期:2020-07-16
中文翻译:

碳酸盐熔体中的稀有气体:通过分子动力学模拟限制溶解度和表面张力
尽管稀有气体(NGs)是地球地幔中的稀有元素,但由于它们的化学惰性,同位素的特异性以及大小变化很大(从He到Rn的质量系数> 50),它们是重要的地球化学示踪剂。当在深度发生部分熔化时,各相之间NG的分配受分布系数控制,该分布系数可由各相中NG的溶解度确定。在这里,我们报告了基于分子动力学模拟的He,Ne,Ar和Xe在碳酸盐熔体中溶解度的定量计算。NG溶解度首先在K 2 CO 3 –CaCO 3中计算混合物在1 bar的压力下与迄今为止唯一可用的实验数据进行了比较。然后,我们研究压力(高达6 GPa)的影响,重点关注两种熔体成分:白云岩和纳碳碳酸盐(模拟坦桑尼亚Ol Doinyo Lengai排放的熔岩)。溶解度随着熔体中碱土金属阳离子的量以及稀有气体的大小而降低。在碳纳米碳熔体中,亨利定律在低压(最高约0.1 GPa)下满足。在较高的压力(几GPa)下,溶解度趋于平稳甚至开始下降(对于He在P > 2 GPa和Ar在P > 4 GPa时)。相反,在熔融白云岩中,压力对所研究的P的影响可忽略不计范围(3–6 GPa)。在上地幔上部的压力下,碳酸盐熔体中NG的溶解度仍与熔融硅酸盐中的NG溶解度相同(10 0至10 1 mol%)。这表明,即使无法忽略与深度成分的依赖关系,并且必须根据具体情况进行评估,深度的碳酸盐熔体也不是NG的优先携带者。最后,我们评估了碳酸盐熔体与天然气之间的界面处的表面张力及其在压力作用下的演化。无论熔体和NG相的成分是什么,当P从0 GPa增加到6 GPa时,表面张力都会增加(约2倍)。此行为与H 2发生时的情况相反O与硅酸盐熔体接触(然后,当压力增加到几GPa时,表面张力下降)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号