当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Inhibitors of Aβ42-Induced Toxicity Have Distinct Metal-Ion Dependency.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-19 , DOI: 10.1021/acschemneuro.0c00192 Ashley J Mason 1 , Ian Hurst 1 , Ravinder Malik 1 , Ibrar Siddique 1 , Inna Solomonov 2 , Irit Sagi 2 , Frank-Gerrit Klärner 3 , Thomas Schrader 3 , Gal Bitan 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-06-19 , DOI: 10.1021/acschemneuro.0c00192 Ashley J Mason 1 , Ian Hurst 1 , Ravinder Malik 1 , Ibrar Siddique 1 , Inna Solomonov 2 , Irit Sagi 2 , Frank-Gerrit Klärner 3 , Thomas Schrader 3 , Gal Bitan 1
Affiliation
|
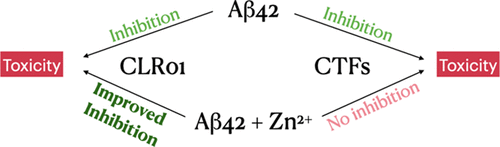
Oligomers of amyloid β-protein (Aβ) are thought to be the proximal toxic agents initiating the neuropathologic process in Alzheimer’s disease (AD). Therefore, targeting the self-assembly and oligomerization of Aβ has been an important strategy for designing AD therapeutics. In parallel, research into the metallobiology of AD has shown that Zn2+ can strongly modulate the aggregation of Aβ in vitro and both promote and inhibit the neurotoxicity of Aβ, depending on the experimental conditions. Thus, successful inhibitors of Aβ self-assembly may have to inhibit the toxicity not only of Aβ oligomers themselves but also of Aβ-Zn2+ complexes. However, there has been relatively little research investigating the effects of Aβ self-assembly and toxicity inhibitors in the presence of Zn2+. Our group has characterized previously a series of Aβ42 C-terminal fragments (CTFs), some of which have been shown to inhibit Aβ oligomerization and neurotoxicity. Here, we asked whether three CTFs shown to be potent inhibitors of Aβ42 toxicity maintained their activity in the presence of Zn2+. Biophysical analysis showed that the CTFs had different effects on oligomer, β-sheet, and fibril formation by Aβ42-Zn2+ complexes. However, cell viability experiments in differentiated PC-12 cells incubated with Aβ42-Zn2+ complexes in the absence or presence of these CTFs showed that the CTFs completely lost their inhibitory activity in the presence of Zn2+ even when applied at 10-fold excess relative to Aβ42. In light of these results, we tested another inhibitor, the molecular tweezer CLR01, which coincidentally had been shown to have a high affinity for Zn2+, suggesting that it could disrupt both Aβ42 oligomerization and Aβ42-Zn2+ complexation. Indeed, we found that CLR01 effectively inhibited the toxicity of Aβ42-Zn2+ complexes. Moreover, it did so at a lower concentration than needed for inhibiting the toxicity of Aβ42 alone. In agreement with these results, CLR01 inhibited β-sheet and fibril formation in Aβ42-Zn2+ complexes. Our data suggest that, for the development of efficient therapeutic agents, inhibitors of Aβ self-assembly and toxicity should be examined in the presence of relevant metal ions and that molecular tweezers may be particularly attractive candidates for therapy development.
中文翻译:

Aβ42 诱导毒性的不同抑制剂具有不同的金属离子依赖性。
淀粉样蛋白 β 蛋白 (Aβ) 的寡聚体被认为是引发阿尔茨海默病 (AD) 神经病理过程的近端毒性剂。因此,靶向 Aβ 的自组装和寡聚化一直是设计 AD 疗法的重要策略。与此同时,对 AD 金属生物学的研究表明,Zn 2+可以在体外强烈调节 Aβ 的聚集,并促进和抑制 Aβ 的神经毒性,具体取决于实验条件。因此,成功的 Aβ 自组装抑制剂可能不仅必须抑制 Aβ 寡聚体本身的毒性,还必须抑制 Aβ-Zn 2+复合物的毒性。然而,在锌存在下研究 Aβ 自组装和毒性抑制剂的影响的研究相对较少。2+。我们小组之前已经表征了一系列 Aβ42 C 末端片段 (CTF),其中一些已被证明可以抑制 Aβ 寡聚化和神经毒性。在这里,我们询问了三种被证明是 Aβ42 毒性的强效抑制剂的 CTF 是否在 Zn 2+存在下保持其活性。生物物理分析表明,CTFs对Aβ42-Zn 2+复合物的低聚物、β-折叠和原纤维形成有不同的影响。然而,与Aβ42-Zn系分化的培养PC-12细胞的细胞活力实验2+在不存在或这些CTFS的存在复合物表明,CTFS完全丧失它们的抑制活性在锌的存在下2+即使以相对于 Aβ42 过量 10 倍的量应用。根据这些结果,我们测试了另一种抑制剂分子镊子 CLR01,巧合的是,它对 Zn 2+具有高亲和力,表明它可以破坏 Aβ42 寡聚化和 Aβ42-Zn 2+复合。事实上,我们发现 CLR01 有效地抑制了 Aβ42-Zn 2+复合物的毒性。此外,它的浓度低于单独抑制 Aβ42 毒性所需的浓度。与这些结果一致,CLR01 抑制了 Aβ42-Zn 2+ 中的β-折叠和原纤维形成复合体。我们的数据表明,为了开发有效的治疗剂,应在相关金属离子存在的情况下检查 Aβ 自组装和毒性的抑制剂,分子镊子可能是治疗开发的特别有吸引力的候选者。
更新日期:2020-08-05
中文翻译:

Aβ42 诱导毒性的不同抑制剂具有不同的金属离子依赖性。
淀粉样蛋白 β 蛋白 (Aβ) 的寡聚体被认为是引发阿尔茨海默病 (AD) 神经病理过程的近端毒性剂。因此,靶向 Aβ 的自组装和寡聚化一直是设计 AD 疗法的重要策略。与此同时,对 AD 金属生物学的研究表明,Zn 2+可以在体外强烈调节 Aβ 的聚集,并促进和抑制 Aβ 的神经毒性,具体取决于实验条件。因此,成功的 Aβ 自组装抑制剂可能不仅必须抑制 Aβ 寡聚体本身的毒性,还必须抑制 Aβ-Zn 2+复合物的毒性。然而,在锌存在下研究 Aβ 自组装和毒性抑制剂的影响的研究相对较少。2+。我们小组之前已经表征了一系列 Aβ42 C 末端片段 (CTF),其中一些已被证明可以抑制 Aβ 寡聚化和神经毒性。在这里,我们询问了三种被证明是 Aβ42 毒性的强效抑制剂的 CTF 是否在 Zn 2+存在下保持其活性。生物物理分析表明,CTFs对Aβ42-Zn 2+复合物的低聚物、β-折叠和原纤维形成有不同的影响。然而,与Aβ42-Zn系分化的培养PC-12细胞的细胞活力实验2+在不存在或这些CTFS的存在复合物表明,CTFS完全丧失它们的抑制活性在锌的存在下2+即使以相对于 Aβ42 过量 10 倍的量应用。根据这些结果,我们测试了另一种抑制剂分子镊子 CLR01,巧合的是,它对 Zn 2+具有高亲和力,表明它可以破坏 Aβ42 寡聚化和 Aβ42-Zn 2+复合。事实上,我们发现 CLR01 有效地抑制了 Aβ42-Zn 2+复合物的毒性。此外,它的浓度低于单独抑制 Aβ42 毒性所需的浓度。与这些结果一致,CLR01 抑制了 Aβ42-Zn 2+ 中的β-折叠和原纤维形成复合体。我们的数据表明,为了开发有效的治疗剂,应在相关金属离子存在的情况下检查 Aβ 自组装和毒性的抑制剂,分子镊子可能是治疗开发的特别有吸引力的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号