当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biophysical Evaluation of Mechanism-Based Probes for Condensation Domains of Nonribosomal Peptide Synthetases.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-06-22 , DOI: 10.1021/acschembio.0c00411 Ce Shi 1 , Bradley R Miller 2 , Evan M Alexander 1 , Andrew M Gulick 2 , Courtney C Aldrich 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-06-22 , DOI: 10.1021/acschembio.0c00411 Ce Shi 1 , Bradley R Miller 2 , Evan M Alexander 1 , Andrew M Gulick 2 , Courtney C Aldrich 1
Affiliation
|
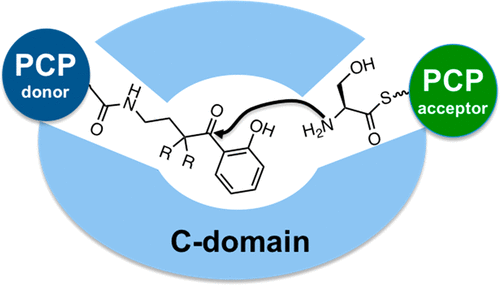
Nonribosomal peptide synthetases (NRPSs) are remarkable modular enzymes that synthesize peptide natural products. The condensation (C) domain catalyzes the key amide bond-forming reaction, but structural characterization with bound donor and acceptor substrates has proven elusive. We describe the chemoenzymatic synthesis of condensation domain probes C1 and C2 designed to cross-link the donor and acceptor substrates within the condensation domain active site. These pantetheine probes contain nonhydrolyzable ketone and α,α-difluoroketone isosteres of the native thioester linkage. Using the bimodular NRPS responsible for synthesis of the siderophore enterobactin as a model system, probe C2 was shown by surface plasmon resonance (SPR) to stabilize an intermolecular interaction between the peptidyl carrier protein (PCP) and C domains in EntB and EntF, respectively, with a dissociation constant of 1–2 nM, whereas the unmodified holo-EntB showed no interaction with EntF. The described condensation domain chemical probes provide powerful tools to study dynamic multifunctional NRPS systems.
中文翻译:

非核糖体肽合成酶缩合域的基于机理的探针的设计,合成和生物物理评估。
非核糖体肽合成酶(NRPS)是合成肽天然产物的出色模块化酶。缩合(C)域催化关键的酰胺键形成反应,但是结合供体和受体底物的结构表征已被证明难以捉摸。我们描述了缩合结构域探针C1和C2的化学酶法合成,设计用于交联在缩合结构域活性位点内的供体和受体底物。这些泛肽探针包含天然硫酯键的不可水解的酮和α,α-二氟酮的等排体。使用负责合成铁载体肠杆菌素的双模块NRPS作为模型系统,探针C2表面等离振子共振(SPR)可以稳定EntB和EntF中肽基载体蛋白(PCP)和C结构域之间的分子间相互作用,解离常数为1-2 nM,而未修饰的holo -EntB则没有与EntF交互。所述缩合域化学探针提供了强大的工具来研究动态多功能NRPS系统。
更新日期:2020-07-17
中文翻译:

非核糖体肽合成酶缩合域的基于机理的探针的设计,合成和生物物理评估。
非核糖体肽合成酶(NRPS)是合成肽天然产物的出色模块化酶。缩合(C)域催化关键的酰胺键形成反应,但是结合供体和受体底物的结构表征已被证明难以捉摸。我们描述了缩合结构域探针C1和C2的化学酶法合成,设计用于交联在缩合结构域活性位点内的供体和受体底物。这些泛肽探针包含天然硫酯键的不可水解的酮和α,α-二氟酮的等排体。使用负责合成铁载体肠杆菌素的双模块NRPS作为模型系统,探针C2表面等离振子共振(SPR)可以稳定EntB和EntF中肽基载体蛋白(PCP)和C结构域之间的分子间相互作用,解离常数为1-2 nM,而未修饰的holo -EntB则没有与EntF交互。所述缩合域化学探针提供了强大的工具来研究动态多功能NRPS系统。









































 京公网安备 11010802027423号
京公网安备 11010802027423号