当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electric Field Measurements Reveal the Pivotal Role of Cofactor-Substrate Interaction in Dihydrofolate Reductase Catalysis.
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-19 , DOI: 10.1021/acscatal.0c01856 Aduragbemi S Adesina 1 , Katarzyna Świderek 2 , Louis Y P Luk 1 , Vicent Moliner 2 , Rudolf K Allemann 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-19 , DOI: 10.1021/acscatal.0c01856 Aduragbemi S Adesina 1 , Katarzyna Świderek 2 , Louis Y P Luk 1 , Vicent Moliner 2 , Rudolf K Allemann 1
Affiliation
|
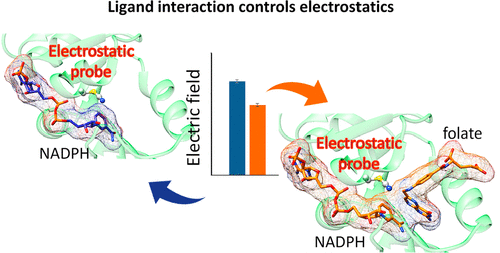
The contribution of ligand–ligand electrostatic interaction to transition state formation during enzyme catalysis has remained unexplored, even though electrostatic forces are known to play a major role in protein functions and have been investigated by the vibrational Stark effect (VSE). To monitor electrostatic changes along important steps during catalysis, we used a nitrile probe (T46C-CN) inserted proximal to the reaction center of three dihydrofolate reductases (DHFRs) with different biophysical properties, Escherichia coli DHFR (EcDHFR), its conformationally impaired variant (EcDHFR-S148P), and Geobacillus stearothermophilus DHFR (BsDHFR). Our combined experimental and computational approach revealed that the electric field projected by the substrate toward the probe negates those exerted by the cofactor when both are bound within the enzymes. This indicates that compared to previous models that focus exclusively on subdomain reorganization and protein–ligand contacts, ligand–ligand interactions are the key driving force to generate electrostatic environments conducive for catalysis.
中文翻译:

电场测量揭示了辅酶-底物相互作用在二氢叶酸还原酶催化中的关键作用。
尽管已知静电作用在蛋白质功能中起主要作用,并且已经通过振动斯塔克效应(VSE)进行了研究,但配体-配体静电相互作用对酶催化过程中过渡态形成的贡献仍未得到探索。为了监测催化过程中重要步骤上的静电变化,我们使用腈探针(T46C-CN)插入了三种具有不同生物物理特性的二氢叶酸还原酶(DHFR)反应中心附近,即大肠杆菌DHFR(EcDHFR),其构象受损的变体( EcDHFR-S148P)和嗜热脂肪地芽孢杆菌DHFR(BsDHFR)。我们的组合实验和计算方法表明,当底物向探针投射的电场与酶结合时,它们抵消了辅因子所施加的电场。这表明,与以前仅专注于子域重组和蛋白质-配体接触的模型相比,配体-配体相互作用是产生有利于催化的静电环境的关键驱动力。
更新日期:2020-07-17
中文翻译:

电场测量揭示了辅酶-底物相互作用在二氢叶酸还原酶催化中的关键作用。
尽管已知静电作用在蛋白质功能中起主要作用,并且已经通过振动斯塔克效应(VSE)进行了研究,但配体-配体静电相互作用对酶催化过程中过渡态形成的贡献仍未得到探索。为了监测催化过程中重要步骤上的静电变化,我们使用腈探针(T46C-CN)插入了三种具有不同生物物理特性的二氢叶酸还原酶(DHFR)反应中心附近,即大肠杆菌DHFR(EcDHFR),其构象受损的变体( EcDHFR-S148P)和嗜热脂肪地芽孢杆菌DHFR(BsDHFR)。我们的组合实验和计算方法表明,当底物向探针投射的电场与酶结合时,它们抵消了辅因子所施加的电场。这表明,与以前仅专注于子域重组和蛋白质-配体接触的模型相比,配体-配体相互作用是产生有利于催化的静电环境的关键驱动力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号