当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conducting Redox Polymer as Organic Anode Material for Polymer‐Manganese Secondary Batteries
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-22 , DOI: 10.1002/celc.202000711 Kouki Oka 1, 2 , Rebecka Löfgren 2 , Rikard Emanuelsson 2 , Hiroyuki Nishide 1 , Kenichi Oyaizu 1 , Maria Strømme 2 , Martin Sjödin 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-22 , DOI: 10.1002/celc.202000711 Kouki Oka 1, 2 , Rebecka Löfgren 2 , Rikard Emanuelsson 2 , Hiroyuki Nishide 1 , Kenichi Oyaizu 1 , Maria Strømme 2 , Martin Sjödin 2
Affiliation
|
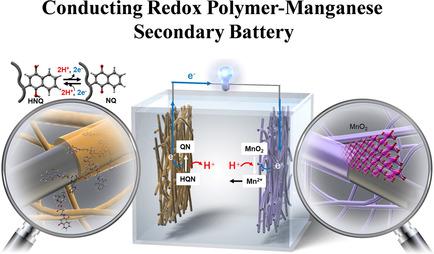
Manganese‐based aqueous batteries have attracted significant attention due to their earth‐abundant components and low environmental burden. However, state‐of‐the‐art manganese‐zinc batteries are poorly rechargeable, owing to dendrite formation on the zinc anode. Organic materials could provide a safe and sustainable replacement. In the present work, a conducting redox polymer (CRP) based on a trimer of EPE (E=3,4‐ethylenedioxythiophene; P=3,4‐propylenedioxythiophene) and a naphthoquinone (NQ) pendant group is used as anode in polymer‐manganese secondary batteries. The polymer shows stable redox conversion around+0.05 V vs. Ag/AgCl, and fast kinetics that involves proton cycling during pendant group redox conversion. For the first time, a CRP‐manganese secondary battery was fabricated with pEP(NQ)E as the anode, manganese oxide as the cathode, and manganese‐containing acidic aqueous solution as the electrolyte. This battery yielded a discharge voltage of 1.0 V and a discharging capacity of 76 mAh/ganode over >50 cycles and high rate capabilities (up to 10 C).
中文翻译:

导电氧化还原聚合物作为聚合物锰二次电池的有机阳极材料
锰基水性电池由于其富含地球的成分和较低的环境负担而备受关注。但是,由于锌阳极上会形成枝晶,因此最先进的锰锌电池的可充电性很差。有机材料可以提供安全,可持续的替代品。在目前的工作中,基于EPE三聚体的导电氧化还原聚合物(CRP)(E = 3,4-亚乙基二氧噻吩; P = 3,4-亚丙基二氧噻吩)和萘醌(NQ)侧基被用作聚合物中的阳极。锰二次电池。相对于Ag / AgCl,该聚合物显示出稳定的氧化还原转化率,相对于Ag / AgCl约为+0.05 V,并且在侧基氧化还原转化过程中涉及质子循环的快速动力学。首次制造了以pEP(NQ)E为阳极,氧化锰为阴极的CRP锰二次电池,以及含锰的酸性水溶液作为电解质。该电池产生1.0V的放电电压和76mAh / g的放电容量阳极经过超过50次循环和高倍率能力(最高10 C)。
更新日期:2020-08-11
中文翻译:

导电氧化还原聚合物作为聚合物锰二次电池的有机阳极材料
锰基水性电池由于其富含地球的成分和较低的环境负担而备受关注。但是,由于锌阳极上会形成枝晶,因此最先进的锰锌电池的可充电性很差。有机材料可以提供安全,可持续的替代品。在目前的工作中,基于EPE三聚体的导电氧化还原聚合物(CRP)(E = 3,4-亚乙基二氧噻吩; P = 3,4-亚丙基二氧噻吩)和萘醌(NQ)侧基被用作聚合物中的阳极。锰二次电池。相对于Ag / AgCl,该聚合物显示出稳定的氧化还原转化率,相对于Ag / AgCl约为+0.05 V,并且在侧基氧化还原转化过程中涉及质子循环的快速动力学。首次制造了以pEP(NQ)E为阳极,氧化锰为阴极的CRP锰二次电池,以及含锰的酸性水溶液作为电解质。该电池产生1.0V的放电电压和76mAh / g的放电容量阳极经过超过50次循环和高倍率能力(最高10 C)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号