当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High‐Voltage Nickel‐Rich NMC Cathode Material with Ionic‐Liquid‐Based Polymer Electrolytes for Rechargeable Lithium‐Metal Batteries
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-20 , DOI: 10.1002/celc.202000608 Himani Gupta 1 , Rajendra Kumar Singh 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-20 , DOI: 10.1002/celc.202000608 Himani Gupta 1 , Rajendra Kumar Singh 1
Affiliation
|
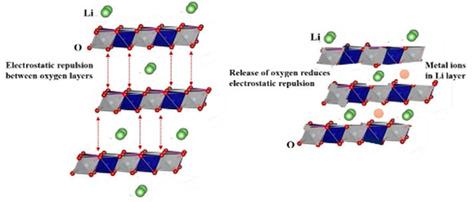
In lithium batteries, high‐nickel‐content layered oxide cathode materials are gaining much attention due to their high capacity and energy density. Therefore, in the present study, a LiNi0.8Mn0.1Co0.1O2 (NMC811) cathode material is synthesized successfully. But, these Ni‐rich cathode materials cannot be operated at high voltage with liquid electrolytes, as they give rise to some problems such as structural instability, high reactivity with electrolyte, electrochemical and thermal instability, and so forth. To suppress these problems, the synthesized ionic‐liquid‐based polymer electrolyte is used over liquid electrolyte as it reduces surface reactivity, enhances cyclic stability and, since it is sufficiently mechanically stable, helps to suppress lithium dendrites growth. Owing to the high electrochemical stability of polymer electrolytes, the performance of the Li battery is also tested at high voltage (4.8 V) and the electrochemical performances are compared at higher and lower cut‐off voltages. The Li battery provides a good capacity (164 mAh.g−1 at C/10) and energy density (611 mWh.g−1) at 4.8 V. In addition, the cyclability of the polymer‐based Li battery is higher, as compared to the liquid‐electrolyte‐based battery. Using the optimized polymer electrolyte, an enhanced structural and interfacial stability of the Li anode and the NMC cathode can be achieved.
中文翻译:

含镍离子液体聚合物电解质的高镍含量的NMC NMC阴极材料,用于可充电锂金属电池
在锂电池中,高镍含量的氧化物正极材料由于其高容量和高能量密度而备受关注。因此,在本研究中,LiNi 0.8 Mn 0.1 Co 0.1 O 2(NMC811)正极材料合成成功。但是,这些富镍阴极材料不能在液态电解质的高压下运行,因为它们会引起一些问题,例如结构不稳定性,与电解质的高反应性,电化学和热不稳定性等。为了抑制这些问题,合成的基于离子液体的聚合物电解质比液体电解质更易使用,因为它降低了表面反应性,增强了循环稳定性,并且由于具有足够的机械稳定性,因此有助于抑制锂树枝状晶体的生长。由于聚合物电解质的高电化学稳定性,锂电池的性能也在高电压(4.8 V)下进行了测试,并且在更高和更低的截止电压下对电化学性能进行了比较。锂电池提供了良好的容量(164 mAh.g-1在C / 10)和能量密度(611 mWh.g -1 4.8 V.此外),基于聚合物的锂电池的循环能力较高,相比于基于液体电解质电池。使用优化的聚合物电解质,可以实现锂阳极和NMC阴极的增强的结构和界面稳定性。
更新日期:2020-06-20
中文翻译:

含镍离子液体聚合物电解质的高镍含量的NMC NMC阴极材料,用于可充电锂金属电池
在锂电池中,高镍含量的氧化物正极材料由于其高容量和高能量密度而备受关注。因此,在本研究中,LiNi 0.8 Mn 0.1 Co 0.1 O 2(NMC811)正极材料合成成功。但是,这些富镍阴极材料不能在液态电解质的高压下运行,因为它们会引起一些问题,例如结构不稳定性,与电解质的高反应性,电化学和热不稳定性等。为了抑制这些问题,合成的基于离子液体的聚合物电解质比液体电解质更易使用,因为它降低了表面反应性,增强了循环稳定性,并且由于具有足够的机械稳定性,因此有助于抑制锂树枝状晶体的生长。由于聚合物电解质的高电化学稳定性,锂电池的性能也在高电压(4.8 V)下进行了测试,并且在更高和更低的截止电压下对电化学性能进行了比较。锂电池提供了良好的容量(164 mAh.g-1在C / 10)和能量密度(611 mWh.g -1 4.8 V.此外),基于聚合物的锂电池的循环能力较高,相比于基于液体电解质电池。使用优化的聚合物电解质,可以实现锂阳极和NMC阴极的增强的结构和界面稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号