当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Eight‐Step Total Synthesis of Pyrroloquinolone‐Type Lycopodium Alkaloid via a Tandem Annulation Approach†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-21 , DOI: 10.1002/cjoc.202000273 Lin‐Rui Zhong 1 , Yuanjie Yang 1 , Bing‐Bing Huang 1 , Zhu‐Jun Yao 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-06-21 , DOI: 10.1002/cjoc.202000273 Lin‐Rui Zhong 1 , Yuanjie Yang 1 , Bing‐Bing Huang 1 , Zhu‐Jun Yao 1
Affiliation
|
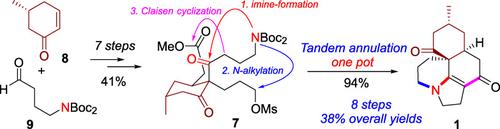
A total synthesis of 6,7,9,10,12,12a‐hexahydro‐2‐methyl‐(2R,4aS,12aS)‐2H,5H‐benzo[e]pyrrolo[3,2,1‐ij]quinolone‐4,11(1H,3H)‐dione (1), a unique pyrroloquinolone‐type Lycopodium alkaloid containing a complex 6/6/6/5 tetracyclic core, has been disclosed in eight steps and 38% overall yield. The reported synthesis features a short, efficient synthesis of the crucial multi‐substituted cyclohexanone intermediate with three stereogenic centers, and one‐pot generation of the complex 6/6/6/5 tetracyclic core in a single tandem step.
中文翻译:

串联法八步合成吡咯并喹诺酮类石蒜生物碱†
6,7,9,10,12,12的总合成一个-六氢-2-甲基- (2 - [R 4如,12 AS)-2 ħ,5 ħ -苯并[ ë ]吡咯并[3,2, 1- IJ ]喹诺酮-4,11-(1 ħ,3 ħ) -二酮(1),一个独特的pyrroloquinolone型石松生物碱含有复合6/6/6/5四环核心,一直在八个步骤和38中公开总产率%。报道的合成方法具有关键的多取代的环己酮中间体的短而有效的合成,该中间体具有三个立体异构中心,并且一步一步即可一次生成复杂的6/6/6/5四环核。
更新日期:2020-06-21
中文翻译:

串联法八步合成吡咯并喹诺酮类石蒜生物碱†
6,7,9,10,12,12的总合成一个-六氢-2-甲基- (2 - [R 4如,12 AS)-2 ħ,5 ħ -苯并[ ë ]吡咯并[3,2, 1- IJ ]喹诺酮-4,11-(1 ħ,3 ħ) -二酮(1),一个独特的pyrroloquinolone型石松生物碱含有复合6/6/6/5四环核心,一直在八个步骤和38中公开总产率%。报道的合成方法具有关键的多取代的环己酮中间体的短而有效的合成,该中间体具有三个立体异构中心,并且一步一步即可一次生成复杂的6/6/6/5四环核。











































 京公网安备 11010802027423号
京公网安备 11010802027423号