当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiamethoxam in aqueous co-solvent mixtures of 1,4-dioxane, N,N-dimethylacetamide, dimethyl sulfoxide and acetonitrile: Solubility solute-solvent and solvent-solvent interactions, and preferential solvation analysis
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106229 Changfei Zhu , Yanyan Zhou , Hongkun Zhao , Ali Farajtabar
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.jct.2020.106229 Changfei Zhu , Yanyan Zhou , Hongkun Zhao , Ali Farajtabar
|
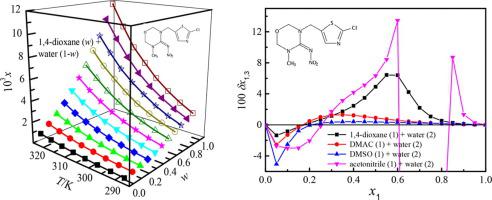
Abstract The determination of equilibrium solubilities of thiamethoxam in solutions of 1,4-dioxane (1) + water (2), N,N-dimethylacetamide (DMAC, 1) + water (2), dimethyl sulfoxide (1) + water (2) and acetonitrile (1) + water (2) was carried out through the shake-flask method from 278.15 to 323.15 K under 101.2 kPa. The relative contributions of solvent-solvent and solute-solvent interactions on the drug solubility variation were studied via the analysis of linear solvation energy relationships. The significant contributions to the solubility variation were the cavity term and dipolarity-polarizability term in aqueous solution of 1,4-dioxane; and cavity term in aqueous solutions of DMAC/DMSO/acetonitrile. The inverse Kirkwood–Buff integrals were employed to investigate the preferential solvation in light of several solution thermodynamic properties. The preferential solvation parameters for 1,4-dioxane, DMAC and acetonitrile presented positive values in the three aqueous solutions within intermediate and 1,4-dioxane/DMAC/acetonitrile-rich compositions, demonstrating that the drug thiamethoxam was solvated preferentially by 1,4-dioxane/DMAC/acetonitrile. It is conjectured that in these regions thiamethoxam serves as a Lewis acid with the 1,4-dioxane and DMAC molecules; while for the (acetonitrile + water) mixture, the preferential solvation could be attributed to polarization effects. Moreover, the solubility data in mole fraction was mathematically described by the van’t Hoff–Jouyban-Acree model and Jouyban-Acree model attaining the average relative deviations no more than 6.67 %.
中文翻译:

噻虫嗪在 1,4-二恶烷、N,N-二甲基乙酰胺、二甲亚砜和乙腈的水性共溶剂混合物中:溶解度溶质-溶剂和溶剂-溶剂相互作用,以及优先溶剂化分析
摘要 噻虫嗪在 1,4-二恶烷 (1) + 水 (2)、N,N-二甲基乙酰胺 (DMAC, 1) + 水 (2)、二甲亚砜 (1) + 水 (2) 溶液中的平衡溶解度测定) 和乙腈 (1) + 水 (2) 通过摇瓶法从 278.15 到 323.15 K 在 101.2 kPa 下进行。通过线性溶剂化能关系分析,研究了溶剂-溶剂和溶质-溶剂相互作用对药物溶解度变化的相对贡献。对溶解度变化的重要贡献是1,4-二恶烷水溶液中的空穴项和偶极-极化项;DMAC/DMSO/乙腈水溶液中的空穴项。根据几种溶液热力学性质,采用逆柯克伍德-布夫积分来研究优先溶剂化。1,4-二恶烷、DMAC 和乙腈的优先溶剂化参数在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先溶剂化为 1,4 -二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。DMAC 和乙腈在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先被 1,4-二恶烷/DMAC/乙腈溶剂化。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。DMAC 和乙腈在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先被 1,4-二恶烷/DMAC/乙腈溶剂化。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。4-二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。4-二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。
更新日期:2020-11-01
中文翻译:

噻虫嗪在 1,4-二恶烷、N,N-二甲基乙酰胺、二甲亚砜和乙腈的水性共溶剂混合物中:溶解度溶质-溶剂和溶剂-溶剂相互作用,以及优先溶剂化分析
摘要 噻虫嗪在 1,4-二恶烷 (1) + 水 (2)、N,N-二甲基乙酰胺 (DMAC, 1) + 水 (2)、二甲亚砜 (1) + 水 (2) 溶液中的平衡溶解度测定) 和乙腈 (1) + 水 (2) 通过摇瓶法从 278.15 到 323.15 K 在 101.2 kPa 下进行。通过线性溶剂化能关系分析,研究了溶剂-溶剂和溶质-溶剂相互作用对药物溶解度变化的相对贡献。对溶解度变化的重要贡献是1,4-二恶烷水溶液中的空穴项和偶极-极化项;DMAC/DMSO/乙腈水溶液中的空穴项。根据几种溶液热力学性质,采用逆柯克伍德-布夫积分来研究优先溶剂化。1,4-二恶烷、DMAC 和乙腈的优先溶剂化参数在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先溶剂化为 1,4 -二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。DMAC 和乙腈在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先被 1,4-二恶烷/DMAC/乙腈溶剂化。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。DMAC 和乙腈在中间体和富含 1,4-二恶烷/DMAC/乙腈的组合物中的三种水溶液中呈现正值,表明药物噻虫嗪优先被 1,4-二恶烷/DMAC/乙腈溶剂化。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。4-二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。4-二恶烷/DMAC/乙腈。据推测,在这些区域,噻虫嗪与 1,4-二恶烷和 DMAC 分子一起作为路易斯酸;而对于(乙腈 + 水)混合物,优先溶剂化可归因于极化效应。此外,摩尔分数中的溶解度数据由 van't Hoff-Jouyban-Acree 模型和 Jouyban-Acree 模型进行数学描述,平均相对偏差不超过 6.67%。










































 京公网安备 11010802027423号
京公网安备 11010802027423号