当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot synthesis of 4-aryl-2-aminothiazoles from styrenes and thioureas promoted by tribromoisocyanuric acid
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-06-21 , DOI: 10.1016/j.tetlet.2020.152164 Vitor S.C. de Andrade , Marcio C.S. de Mattos
中文翻译:

由三溴异氰尿酸促进的苯乙烯和硫脲的一锅合成4-芳基-2-氨基噻唑
更新日期:2020-07-05
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-06-21 , DOI: 10.1016/j.tetlet.2020.152164 Vitor S.C. de Andrade , Marcio C.S. de Mattos
|
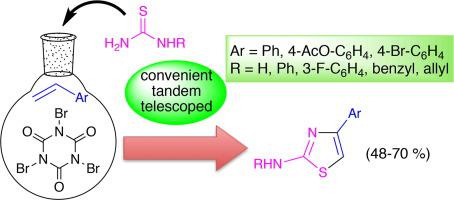
A simple and efficient one-pot protocol has been developed for the conversion of styrenes into 4-aryl-2-aminothiazoles using readily available starting materials. Tribromoisocyanuric acid was successfully used for the co-bromination and oxidation of styrenes to give phenacyl bromides, which in the presence of thioureas produced the corresponding 4-aryl-2-aminothiazoles in 48–70% yield. The protocol involves three reactions in one process: a tandem (formation of phenacyl bromides from styrenes) followed by a telescoped (conversion to thiazole) reaction.
中文翻译:

由三溴异氰尿酸促进的苯乙烯和硫脲的一锅合成4-芳基-2-氨基噻唑
已经开发出一种简单有效的一锅法,用于使用容易获得的起始原料将苯乙烯转化为4-芳基-2-氨基噻唑。三溴异氰尿酸已成功用于苯乙烯的共溴化和氧化,得到苯甲酰溴,在硫脲存在下,苯甲酰苯能以48-70%的产率产生相应的4-芳基-2-氨基噻唑。该方案在一个过程中涉及三个反应:串联(由苯乙烯形成苯甲酰溴),然后进行伸缩(转化为噻唑)反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号