Parasitology International ( IF 1.5 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.parint.2020.102160 Akira Nozawa 1 , Daisuke Ito 2 , Mohamed Ibrahim 3 , Herbert J Santos 4 , Takafumi Tsuboi 1 , Yuzuru Tozawa 5
|
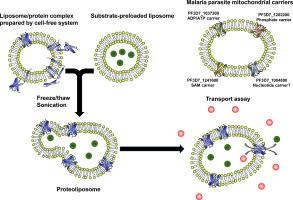
Members of the mitochondrial carrier (MC) family of membrane transporters play important roles in cellular metabolism. We previously established an in vitro reconstitution system for membrane transporters based on wheat germ cell-free translation system. We have now applied this reconstitution system to the comparative analysis of MC proteins from the malaria parasite Plasmodium falciparum and Saccharomyces cerevisiae. We synthesized twelve putative P. falciparum MCs and determined the transport activities of four of these proteins including PF3D7_1037300 protein (ADP/ATP translocator), PF3D7_1004800 protein (ADP/ATP translocator), PF3D7_1202200 protein (phosphate carrier), and PF3D7_1241600 protein (S-adenosylmethionine transporter). In addition, we tested the effect of cardiolipin on the activity of MC proteins. The transport activities of the yeast MCs, ScAac2p, ScGgc1p, ScDic1p, ScPic1p, and ScSam5p, which localize to the mitochondrial inner membrane, were increased by cardiolipin supplementation, whereas that of ScAnt1p, which localizes to the peroxisome membrane, was not significantly affected. Together, this indicates that the functional properties of the reconstituted MCs reflect the lipid content of their native membranes. Except for PF3D7_1241600 protein, these P. falciparum proteins manifested cardiolipin-dependent transport activities. Immunofluorescence analysis showed that PF3D7_1241600 protein is not mainly localized to the mitochondria of P. falciparum cells. We thus revealed the functions of four MC proteins of the malaria parasite and the effects of cardiolipin on their activities.
中文翻译:

基于体外翻译和重组表征疟原虫恶性疟原虫的线粒体载体蛋白。
膜转运蛋白的线粒体载体(MC)家族成员在细胞代谢中起重要作用。我们以前建立了基于小麦无细胞翻译系统的膜转运蛋白的体外重组系统。我们现在已经将这种重组系统应用于疟原虫恶性疟原虫和酿酒酵母中MC蛋白的比较分析。我们合成了十二种假定的恶性疟原虫MC,并确定了其中四种蛋白的转运活性,包括PF3D7_1037300蛋白(ADP / ATP转运子),PF3D7_1004800蛋白(ADP / ATP转运子),PF3D7_1202200蛋白(磷酸盐载体)和PF3D7_1241600蛋白(S-腺苷蛋氨酸转运蛋白)。此外,我们测试了心磷脂对MC蛋白活性的影响。补充心磷脂可以提高定位于线粒体内膜的酵母MC,ScAac2p,ScGgc1p,ScDic1p,ScPic1p和ScSam5p的转运活性,而对定位于过氧化物酶体膜的ScAnt1p的转运活性则没有显着影响。总之,这表明重构的MC的功能特性反映了其天然膜的脂质含量。除PF3D7_1241600蛋白外,这些恶性疟原虫蛋白还表现出心磷脂依赖性转运活性。免疫荧光分析表明PF3D7_1241600蛋白并非主要定位于恶性疟原虫的线粒体细胞。因此,我们揭示了疟原虫的四种MC蛋白的功能以及心磷脂对其活性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号