当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins
Nano Today ( IF 13.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.nantod.2020.100898 Armin Vedadghavami 1 , Chenzhen Zhang 1 , Ambika G Bajpayee 1, 2
Nano Today ( IF 13.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.nantod.2020.100898 Armin Vedadghavami 1 , Chenzhen Zhang 1 , Ambika G Bajpayee 1, 2
Affiliation
|
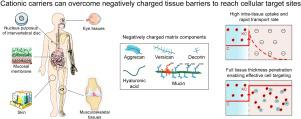
Negatively charged tissues are ubiquitous in the human body and are associated with a number of common diseases yet remain an outstanding challenge for targeted drug delivery. While the anionic proteoglycans are critical for tissue structure and function, they make tissue matrix dense, conferring a high negative fixed charge density (FCD) that makes drug penetration through the tissue deep zones and drug delivery to resident cells extremely challenging. The high negative FCD of these tissues is now being utilized by taking advantage of electrostatic interactions to create positively charged multi-stage delivery methods that can sequentially penetrate through the full thickness of tissues, create a drug depot and target cells. After decades of work on attempting delivery using strong binding interactions, significant advances have recently been made using weak and reversible electrostatic interactions, a characteristic now considered essential to drug penetration and retention in negatively charged tissues. Here we discuss these advances using examples of negatively charged tissues (cartilage, meniscus, tendons and ligaments, nucleus pulposus, vitreous of eye, mucin, skin), and delve into how each of their structures, tissue matrix compositions and high negative FCDs create barriers to drug entry and explore how charge interactions are being used to overcome these barriers. We review work on tissue targeting cationic peptide and protein-based drug delivery, compare and contrast drug delivery designs, and also present examples of technologies that are entering clinical trials. We also present strategies on further enhancing drug retention within diseased tissues of lower FCD by using synergistic effects of short-range binding interactions like hydrophobic and H-bonds that stabilize long-range charge interactions. As electrostatic interactions are incorporated into design of drug delivery materials and used as a strategy to create properties that are reversible, tunable and dynamic, bio-electroceuticals are becoming an exciting new direction of research and clinical work.
中文翻译:

克服带负电的组织屏障:使用阳离子肽和蛋白质进行药物输送
带负电的组织在人体内普遍存在,并与许多常见疾病相关,但仍然是靶向药物递送的一个突出挑战。虽然阴离子蛋白聚糖对组织结构和功能至关重要,但它们使组织基质致密,赋予高负固定电荷密度 (FCD),这使得药物渗透组织深层并将药物递送至驻留细胞极具挑战性。现在,通过利用静电相互作用来利用这些组织的高负 FCD,创建带正电荷的多级递送方法,该方法可以顺序穿透组织的整个厚度,创建药物库和靶细胞。经过数十年尝试使用强结合相互作用进行递送的工作,最近在使用弱且可逆的静电相互作用方面取得了重大进展,这一特性现在被认为对于药物在带负电的组织中渗透和保留至关重要。在这里,我们使用带负电荷的组织(软骨、半月板、肌腱和韧带、髓核、眼睛玻璃体、粘蛋白、皮肤)的例子来讨论这些进展,并深入研究它们的每种结构、组织基质成分和高负 FCD 如何形成障碍药物进入并探索如何利用电荷相互作用来克服这些障碍。我们回顾了组织靶向阳离子肽和基于蛋白质的药物递送的工作,比较和对比药物递送设计,并介绍了正在进入临床试验的技术示例。 我们还提出了通过利用短程结合相互作用(如稳定长程电荷相互作用的疏水键和氢键)的协同效应,进一步增强药物在较低 FCD 的病变组织内保留的策略。随着静电相互作用被纳入药物输送材料的设计并用作创造可逆、可调和动态特性的策略,生物电子药物正在成为令人兴奋的研究和临床工作新方向。
更新日期:2020-10-01
中文翻译:

克服带负电的组织屏障:使用阳离子肽和蛋白质进行药物输送
带负电的组织在人体内普遍存在,并与许多常见疾病相关,但仍然是靶向药物递送的一个突出挑战。虽然阴离子蛋白聚糖对组织结构和功能至关重要,但它们使组织基质致密,赋予高负固定电荷密度 (FCD),这使得药物渗透组织深层并将药物递送至驻留细胞极具挑战性。现在,通过利用静电相互作用来利用这些组织的高负 FCD,创建带正电荷的多级递送方法,该方法可以顺序穿透组织的整个厚度,创建药物库和靶细胞。经过数十年尝试使用强结合相互作用进行递送的工作,最近在使用弱且可逆的静电相互作用方面取得了重大进展,这一特性现在被认为对于药物在带负电的组织中渗透和保留至关重要。在这里,我们使用带负电荷的组织(软骨、半月板、肌腱和韧带、髓核、眼睛玻璃体、粘蛋白、皮肤)的例子来讨论这些进展,并深入研究它们的每种结构、组织基质成分和高负 FCD 如何形成障碍药物进入并探索如何利用电荷相互作用来克服这些障碍。我们回顾了组织靶向阳离子肽和基于蛋白质的药物递送的工作,比较和对比药物递送设计,并介绍了正在进入临床试验的技术示例。 我们还提出了通过利用短程结合相互作用(如稳定长程电荷相互作用的疏水键和氢键)的协同效应,进一步增强药物在较低 FCD 的病变组织内保留的策略。随着静电相互作用被纳入药物输送材料的设计并用作创造可逆、可调和动态特性的策略,生物电子药物正在成为令人兴奋的研究和临床工作新方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号