Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.yjmcc.2020.06.006 Michael J Daseke 1 , Mavis A A Tenkorang-Impraim 2 , Yonggang Ma 3 , Upendra Chalise 4 , Shelby R Konfrst 4 , Michael R Garrett 5 , Kristine Y DeLeon-Pennell 6 , Merry L Lindsey 4
|
Introduction
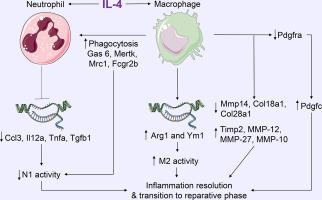
Macrophages and neutrophils are primary leukocytes involved in the inflammatory response to myocardial infarction (MI). While interleukin (IL)-4 is an in vitro anti-inflammatory stimulus, the MI myocardium does not express a considerable amount of IL-4 but does express IL4 receptors. We hypothesized that continuous exogenous IL-4 infusion starting 24 h after MI would promote a polarization switch in inflammatory cells towards a reparative phenotype.
Methods
C57BL/6J male mice (3–6 months of age) were subcutaneously infused with either saline (n = 17) or IL-4 (20 ng/g/day; n = 17) beginning 24 h after MI and evaluated at MI day 3.
Results
Macrophages and neutrophils were isolated ex vivo from the infarct region and examined. Exogenous IL-4 decreased pro-inflammatory Ccl3, Il12a, Tnfa, and Tgfb1 in neutrophils and increased anti-inflammatory Arg1 and Ym1 in macrophages (all p < .05). Tissue clearance by IL-4 treated neutrophils was not different, while selective phagocytosis of neutrophils doubled in IL-4 treated macrophages (p < .05). Of 24,339 genes examined by RNA-sequencing, 2042 genes were differentially expressed in macrophages from IL-4 stimulated infarct (all FDR p < .05). Pdgfc gene expression was ranked first, increasing 3-fold in macrophages stimulated with IL-4 (p = 1 × 10−9). Importantly, changes in macrophage physiology and transcriptome occurred in the absence of global LV effects. Bone marrow derived monocytes stimulated with mouse recombinant PDGF-CC protein (10 μg/ml) or PDGF-CC blocking antibody (200 ng/ml) did not change Arg1 or Ym1 expression, indicating the in vivo effect of IL-4 to stimulate macrophage anti-inflammatory gene expression was independent of PDGF-CC.
Conclusions
Our results indicate that exogenous IL-4 promotes inflammation resolution by turning off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to mediate removal of apoptotic neutrophils.
中文翻译:

外源性 IL-4 可以阻断中性粒细胞的促炎症反应,同时刺激巨噬细胞的抗炎作用,从而诱导心肌梗死后的中性粒细胞吞噬作用。
介绍
巨噬细胞和中性粒细胞是参与心肌梗死 (MI) 炎症反应的主要白细胞。虽然白细胞介素 (IL)-4 是一种体外抗炎刺激物,但 MI 心肌不表达大量的 IL-4,但表达 IL4 受体。我们假设,MI 后 24 小时开始持续外源性 IL-4 输注会促进炎症细胞向修复表型极化转变。
方法
MI 后 24 小时开始,C57BL/6J 雄性小鼠(3-6 月龄)皮下注射生理盐水 ( n = 17) 或 IL-4(20 ng/g/天;n = 17),并在 MI 当天进行评估3.
结果
从梗塞区域离体分离巨噬细胞和中性粒细胞并进行检查。外源性 IL-4 降低了中性粒细胞中的促炎性Ccl3 、 Il12a 、 Tnfa和Tgfb1 ,并增加了巨噬细胞中的抗炎性 Arg1 和 Ym1(全部p < .05)。 IL-4 处理的中性粒细胞的组织清除没有差异,而 IL-4 处理的巨噬细胞中中性粒细胞的选择性吞噬作用加倍 ( p < .05)。在通过 RNA 测序检查的 24,339 个基因中,2042 个基因在 IL-4 刺激的梗塞巨噬细胞中差异表达(所有 FDR p < .05)。 Pdgfc基因表达排名第一,在 IL-4 刺激的巨噬细胞中增加了 3 倍 ( p = 1 × 10 -9 )。重要的是,巨噬细胞生理学和转录组的变化是在没有整体左心室效应的情况下发生的。用小鼠重组 PDGF-CC 蛋白 (10 μg/ml) 或 PDGF-CC 阻断抗体 (200 ng/ml) 刺激的骨髓来源的单核细胞没有改变 Arg1 或 Ym1 表达,表明 IL-4 刺激巨噬细胞的体内作用抗炎基因表达与PDGF-CC无关。
结论
我们的结果表明,外源性 IL-4 通过关闭中性粒细胞中的促炎症同时刺激巨噬细胞中的抗炎症介导凋亡中性粒细胞的去除来促进炎症消退。











































 京公网安备 11010802027423号
京公网安备 11010802027423号