Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.jcat.2020.06.014 James W. Harris , Anuj A. Verma , Jeremy W. Arvay , Arthur J. Shih , W. Nicholas Delgass , Fabio H. Ribeiro
|
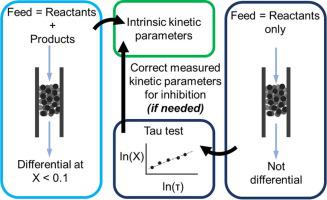
While the potential for product inhibition in catalytic reactions is well known, the impact of neglected inhibition on measured kinetic parameters is often overlooked. The presence of product inhibition, most often caused by the competitive adsorption of products with reactants on catalytic active sites, is difficult to determine a priori for an arbitrary catalytic system. The significance of product inhibition relies on the concentration of the products, their adsorption thermodynamics on catalytically relevant sites, and process parameters such as temperature and pressure. When inhibition is significant, however, apparent activation energies and reaction orders vary from the differential-reactor apparent activation energy by a factor of 1/(1 − δ), where δ is the total inhibition order (e.g., the factor (1 − δ) = 1.6 for a system with product inhibition of −0.6 order). This is illustrated here with the kinetics of NO oxidation over Cu ion clusters (CuxOy) in Cu-SSZ-13, for which the product NO2 inhibits the forward reaction. Furthermore, in the presence of inhibition, when only reactants are fed to a flow reactor or placed in a batch reactor, there is often no practical conversion that is low enough to guarantee differential behavior. Inclusion of products in the feed solves this problem, allowing accurate determination of kinetic parameters such as apparent reaction orders and activation energies. We also demonstrate that evaluation of the necessity of co-feeding products to assure measurement of differential-reactor data in a given catalytic system is straightforward from a plot of the log of the rate (or conversion) versus the log of the space time in a flow reactor or elapsed time in a batch reactor. We encourage inclusion of this test in all kinetic analyses that are reasonably approximated by power law rate expressions.
中文翻译:

动力学参数定量中产物抑制的后果
尽管催化反应中产物抑制的潜力是众所周知的,但忽略的抑制对测得的动力学参数的影响常常被忽略。产品抑制作用的存在(通常是由产品与反应物在催化活性位点上的竞争性吸附引起的)很难先验确定用于任意催化系统。产物抑制的重要性取决于产物的浓度,它们在催化相关位点上的吸附热力学以及工艺参数,例如温度和压力。但是,当抑制作用显着时,表观活化能和反应阶数与微分反应器的表观活化能相差1 /(1-δ),其中δ是总抑制阶数(例如,因子(1-δ )= 1.6(对于产品抑制为-0.6级的系统)。这在此处用NO-在Cu-SSZ-13中的Cu离子簇(Cu x O y)上的氧化动力学来说明,其产物为NO 2抑制正向反应。此外,在存在抑制作用的情况下,当仅将反应物进料至流动反应器或置于间歇反应器中时,通常没有足够低的实际转化率来保证差异行为。进料中包含产品可以解决此问题,从而可以准确确定动力学参数,例如表观反应顺序和活化能。我们还证明,从速率(或转化率)对数与时空对数的关系图中可以很容易地评估为确保给定催化系统中差动反应器数据的测量而需要进料的评估。流式反应器或间歇式反应器中经过的时间。我们鼓励将此检验包括在所有通过幂律速率表达式合理近似的动力学分析中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号