International Journal for Parasitology ( IF 4 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.ijpara.2020.03.015 Adam P S Bennett 1 , Eduardo de la Torre-Escudero 1 , Nicola A M Oliver 1 , Kathryn M Huson 1 , Mark W Robinson 1
|
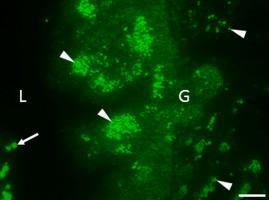
Parasitic helminths secrete extracellular vesicles (EVs) which have potent immunomodulatory effects. Whilst the cargo of EVs has been characterised for many species, we know little about the mechanisms that govern their biogenesis and release. Using antibodies raised against a panel of Fasciola hepatica EV (FhEV) marker proteins, we have identified multiple sites of EV production in the parasite. Discrete immunofluorescence patterns were observed within the gastrodermal cells and tegumental syncytium for different marker proteins whilst the protonephridial (excretory) system and parenchymal-type 2 cells were identified as additional sites of production (or transit) of FhEVs. Ligation was used to mechanically block the oral sucker, excretory pore, or both, to determine the effect on FhEV release from live adult flukes in vitro. This revealed that FhEVs are predominately derived from the gut, whilst the tegument releases EVs to a lesser extent. The data also suggest that the protonephridial system contributes to the small (120 K) EV sub-population. Sphingomyelinase (SMase) activity is a key driver of EV biogenesis in mammalian cells and we have previously identified SMases in FhEVs by mass spectrometry. SMase activity associated with isolated FhEVs was susceptible to the chemical inhibitor GW4869 and treatment of adult flukes with GW4869 led to a significant reduction in 120 K EV release in vitro, suggesting that a ceramide-dependent mechanism could drive 120 K EV formation. In contrast, the release of the larger 15 K EVs was only moderately impacted, indicating that they form independently of SMase activity. Ultrastructural observation of GW4869-treated F. hepatica tissue showed severe disruption to the parenchyma and vacuolation of the tegument, gastrodermal cells and epithelial lining of the excretory ducts. This work establishes that targeted disruption of EV biogenesis and release in helminths is possible, and provides proof-of-concept for future studies investigating EV secretion as a target for parasite control.
中文翻译:

蠕虫病原体Fasciola hepatica释放的细胞外囊泡的细胞和分子起源。
寄生性蠕虫分泌具有有效免疫调节作用的细胞外囊泡(EVs)。尽管电动汽车的货源已经针对许多物种进行了表征,但我们对其控制其生物发生和释放的机制知之甚少。使用针对一组Fasciola hepatica EV(Fh EV)标记蛋白的抗体,我们在寄生虫中鉴定了多个EV产生位点。在胃真皮细胞和皮膜合胞体中观察到不同标记蛋白的离散免疫荧光模式,而前肾上腺(排泄)系统和实质2型细胞被鉴定为Fh的其他生产(或转运)位点电动汽车。结扎用于机械阻塞口腔吸盘,排泄孔或两者,以确定对活体成年吸虫释放Fh EV的影响。这表明,Fh EV主要来自肠道,而皮被释放的程度较小。数据还表明,前肾上腺系统有助于较小的(120 K)EV亚人群。鞘磷脂酶(SMase)活性是哺乳动物细胞中EV生物发生的关键驱动力,我们之前已经通过质谱法鉴定了Fh EV中的SMase 。与孤立的Fh相关的SMase活性电动汽车易受化学抑制剂GW4869的影响,用GW4869处理成人吸虫可导致体外120 K EV释放显着减少,这表明神经酰胺依赖性机制可推动120 K EV的形成。相反,较大的15 K EV的释放仅受到中度影响,表明它们的形成独立于SMase活性。GW4869处理的F.肝组织的超微结构观察显示,严重破坏了实质,并覆盖了肠壁,胃真皮细胞和排泄导管的上皮衬层。这项工作确立了可能有针对性地破坏EV的生物发生和释放蠕虫的行为,并为将EV分泌作为寄生虫控制目标的未来研究提供了概念验证。



























 京公网安备 11010802027423号
京公网安备 11010802027423号