Electrochimica Acta ( IF 5.5 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.electacta.2020.136620 Valentín Briega-Martos , Adolfo Ferre-Vilaplana , Enrique Herrero , Juan M. Feliu
|
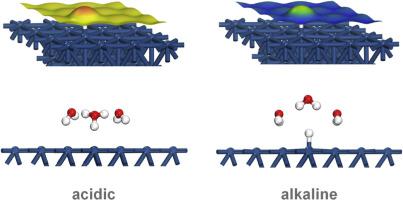
Platinum is a very effective electrode for the hydrogen evolution and oxidation reactions (HER/HOR) in acidic media. However, the activity for the HOR on platinum falls two orders of magnitude from acidic to alkaline media, which has not been completely understood yet. Here, we provide an explanation for that. Both the HER and the HOR were investigated on the three basal planes of platinum in a pH range near neutral pH conditions in buffered solutions in the absence of anion specific adsorption for guaranteeing the protons availability. Whereas changes in the pH from acid to neutral values produced negligible effects on the HER, the HOR was found to be pH sensitive, even under near neutral pH conditions. From these results, it can be consistently reasoned that the drastic fall in the activity of the HOR on platinum from acidic to alkaline media is an effect of the charge on the electrode, which is more negative as the pH increases. With the aid of density functional theory calculations, kinetic arguments explaining the unfavorable effect that negative charge on the electrode has on the HOR are provided.
中文翻译:

为什么随着pH升高,氢氧化反应在铂上的活性降低
铂是用于在酸性介质中进行氢释放和氧化反应(HER / HOR)的非常有效的电极。但是,从酸性介质到碱性介质,HOR在铂上的活性下降了两个数量级,目前尚未完全了解。在此,我们对此进行解释。在没有阴离子特异性吸附来保证质子可用性的情况下,在缓冲溶液中接近中性pH条件的pH范围内,在铂的三个基面上对HER和HOR进行了研究。尽管pH值从酸值变为中性值对HER的影响可忽略不计,但发现HOR对pH敏感,即使在接近中性的pH条件下也是如此。根据这些结果,可以一贯地认为,HOR对铂的活性从酸性到碱性介质的急剧下降是电极上电荷的影响,随着pH的增加,这种影响更为不利。借助于密度泛函理论计算,提供了解释电极上的负电荷对HOR的不利影响的动力学论据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号