Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.bbamem.2020.183402 Zanxia Cao 1 , Lei Liu 2 , Guodong Hu 1 , Yunqiang Bian 1 , Haiyan Li 3 , Jihua Wang 1 , Yaoqi Zhou 4
|
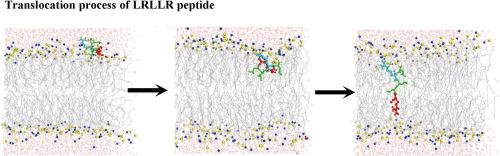
Spontaneous Membrane Translocating Peptides (SMTPs) can translocate silently across the bilayer and, thus, have the best potential to improve the delivery of therapeutic molecules to cells without toxicity. However, how their translocation mechanisms are affected by a specific peptide sequence remains poorly understood. Here, bias-exchange metadynamics simulations were employed to investigate the translocation mechanisms of five SMTPs with the same composition of amino acids (LLRLR, LRLLR, LLLRR, RLLLR, and LRLRL). Simulation results yield sequence-dependent free energy barrier using the FESs along the z-directional distance. An in-depth analysis of sequence-dependent interactions in different regions of the bilayers indicates that the free-energy barrier height of a specific sequence is resulted from the accessibility balance of isolated or clustered hydrophobic residues (L) and hydrophilic residues (R) that leads to different levels of resistance for moving of a peptide into the hydrophobic center of the membrane. At the maximal of the free-energy barrier, all peptides have a conformation parallel to the membrane surface with the barrier height determined by their affinity to the hydrophobic region. The appropriate bilayer perturbation and GDM+ pairing are beneficial for peptide translocation. These results provide an improved microscopic understanding of how peptide sequence influences the translocation efficiency and mechanism.
中文翻译:

偏差交换元动力学模拟揭示了自发膜转运肽的序列依赖性细胞穿透中疏水和亲水相互作用的相互作用。
自发膜转运肽(SMTPs)可以在双层中静默转运,因此,具有最佳的潜力,可以改善治疗分子向细胞的输送而无毒性。但是,它们的易位机制如何受特定的肽序列影响仍然知之甚少。在这里,使用偏向交换元动力学模拟来研究具有相同氨基酸组成(LLRLR,LRLLR,LLLRR,RLLLR和LRLRL)的五个SMTP的转运机制。仿真结果使用沿Z方向距离的FES产生依赖于序列的自由能垒。对双层不同区域中依赖序列的相互作用的深入分析表明,特定序列的自由能垒高度是由于分离或成簇的疏水性残基(L)和亲水性残基(R)的可及性平衡引起的。导致将肽移动到膜的疏水中心的抗性水平不同。在自由能屏障的最大值处,所有肽均具有平行于膜表面的构象,且屏障高度取决于它们对疏水区的亲和力。适当的双层摄动和GDM 所有的肽都具有平行于膜表面的构象,其屏障高度取决于它们对疏水区的亲和力。适当的双层摄动和GDM 所有的肽都具有平行于膜表面的构象,其屏障高度取决于它们对疏水区的亲和力。适当的双层摄动和GDM+配对有利于肽转运。这些结果提供了对肽序列如何影响转运效率和机制的改进的微观理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号