Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.apcata.2020.117712 Yanan Gao , Long Zhang , Arno J.F. van Hoof , Emiel J.M. Hensen
|
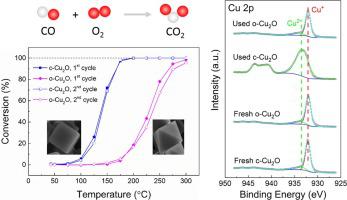
Solid materials can exhibit very different surface catalytic properties depending on the exposed surface. Different from the previous finding by Bao et al. (Angew. Chem. Int. Ed. 50 (2011) 12294–12298) that Cu2O octahedra are more active than Cu2O cubes in CO oxidation, we found that Cu2O cubes exposing {100} surface showed significantly higher activity than Cu2O octahedra exposing {111} surface in the reaction. The Cu2O(100) surface was found to be oxidized during CO oxidation, leading to the formation of a CuO surface layer. On the other hand, Cu2O(111) strongly resisted such surface oxidation under CO oxidation conditions. Density functional theory calculations demonstrate that CO oxidation follows a Mars-Van Krevelen mechanism on Cu2O(111) with a high activation barrier of 1.36 eV. In contrast, the overall activation barrier is only 0.58 eV for CuO(111). These differences explain well the higher activity of oxidized Cu2O cubes.
中文翻译:

关于CO氧化过程中Cu 2 O的表面依赖性氧化:Cu 2+比Cu +更具活性
固体材料可根据暴露的表面表现出非常不同的表面催化性能。与Bao等人先前的发现不同。(Angew。Chem。Int。Ed。50(2011)12294–12298),在CO氧化中,Cu 2 O八面体比Cu 2 O立方体更具活性,我们发现暴露于{100}表面的Cu 2 O立方体表现出明显更高的活性。比Cu 2 O八面体在反应中暴露{111}表面。发现Cu 2 O(100)表面在CO氧化过程中被氧化,导致形成了CuO表面层。另一方面,Cu 2O(111)在CO氧化条件下强烈抵抗这种表面氧化。密度泛函理论计算表明,CO氧化如下对Cu一个火星范雷维伦机构2 O(111)与1.36电子伏特的高激活的屏障。相反,CuO(111)的总激活势垒仅为0.58 eV。这些差异很好地解释了氧化的Cu 2 O立方体的更高活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号