当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A continuous-flow approach for the multi-gram scale synthesis of C2-alkyl- or β-amino functionalized 1,3-dicarbonyl derivatives and ondansetron drug using 1,3-dicarbonyls
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0re00171f Nirmala Mohanta 1, 2, 3, 4 , Krishna Nair 1, 2, 3, 4 , Dasharath Vishambar Sutar 1, 2, 3, 4 , Boopathy Gnanaprakasam 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-06-19 , DOI: 10.1039/d0re00171f Nirmala Mohanta 1, 2, 3, 4 , Krishna Nair 1, 2, 3, 4 , Dasharath Vishambar Sutar 1, 2, 3, 4 , Boopathy Gnanaprakasam 1, 2, 3, 4
Affiliation
|
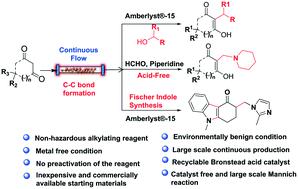
Continuous-flow chemistry is a modern technology that encompasses the green chemistry principles for the multi-gram synthesis of various API and drugs. Herein, we have developed a highly efficient and environmentally benign metal-free alkylation of 1,3-dicarbonyl compounds using secondary alcohols in the presence of inexpensive Amberlyst®-15 under continuous-flow. This method has a broad substrate scope with a variety of secondary alcohols and water as a byproduct. The Amberlyst®-15 is recyclable and reusable for the alkylation reaction under batch/continuous-flow technology. Furthermore, a continuous-flow technology driven Mannich reaction was demonstrated under an acid-free condition. In addition, a continuous-flow Fischer indole strategy for the ondansetron with an improved yield was demonstrated. Additionally, all these reactions were demonstrated with multi-gram scale synthesis without lowering the yield under batch/continuous-flow technology.
中文翻译:

使用1,3-二羰基化合物连续克数级合成C2-烷基或β-氨基官能化的1,3-二羰基衍生物和恩丹西酮药物的连续流方法
连续流化学是一种现代技术,涵盖了绿色化学原理,可用于多种API和药物的多克合成。本文中,我们在廉价的Amberlyst®-15存在下,在连续流动条件下,使用仲醇开发了一种高效且对环境无害的1,3-二羰基化合物无金属烷基化反应。该方法具有广泛的底物范围,副产物包括各种仲醇和水。-15是可回收的,可在间歇/连续流技术下用于烷基化反应。此外,在无酸条件下证明了采用连续流技术驱动的曼尼希反应。此外,还证明了用于恩丹西酮的连续流费歇尔吲哚策略具有提高的产率。另外,
更新日期:2020-07-28
中文翻译:

使用1,3-二羰基化合物连续克数级合成C2-烷基或β-氨基官能化的1,3-二羰基衍生物和恩丹西酮药物的连续流方法
连续流化学是一种现代技术,涵盖了绿色化学原理,可用于多种API和药物的多克合成。本文中,我们在廉价的Amberlyst®-15存在下,在连续流动条件下,使用仲醇开发了一种高效且对环境无害的1,3-二羰基化合物无金属烷基化反应。该方法具有广泛的底物范围,副产物包括各种仲醇和水。-15是可回收的,可在间歇/连续流技术下用于烷基化反应。此外,在无酸条件下证明了采用连续流技术驱动的曼尼希反应。此外,还证明了用于恩丹西酮的连续流费歇尔吲哚策略具有提高的产率。另外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号