当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allylboronates from Vinyl Triflates and α-Chloroboronates by Reductive Nickel Catalysis.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.orglett.0c01683 Jin-Bao Qiao 1 , Zhen-Zhen Zhao 1 , Ya-Qian Zhang 1 , Kai Yin 2 , Zhi-Xiong Tian 1 , Xing-Zhong Shu 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.orglett.0c01683 Jin-Bao Qiao 1 , Zhen-Zhen Zhao 1 , Ya-Qian Zhang 1 , Kai Yin 2 , Zhi-Xiong Tian 1 , Xing-Zhong Shu 1
Affiliation
|
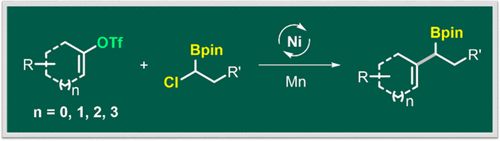
Allylboronates are unique building blocks widely used in organic synthesis, but the construction of cyclic allylboranates remains a challenging subject. We demonstrate here a mild and efficient access to this type of compound through the cross-electrophile coupling of vinyl triflates and α-chloroboronates. The reaction proceeded with a good substrate scope and good functional group compatibility. The ready availability of vinyl triflates from ketones, as well as the rich chemistry of allylboranates, makes our method suitable for the divergent modification of biologically active compounds. Preliminary mechanistic studies revealed that α-chloroboronates were activated via a radical process.
中文翻译:

通过还原镍催化,从乙烯基三氟甲磺酸酯和α-氯硼酸酯中得到烯丙基硼酸酯。
烯丙基硼酸酯是广泛用于有机合成的独特构建基,但是环状烯丙基硼酸酯的构建仍然是一个具有挑战性的课题。我们在这里展示了通过三氟甲磺酸酯和α-氯硼酸酯的交叉亲电子偶联,温和而有效地访问此类化合物的方法。反应以良好的底物范围和良好的官能团相容性进行。酮中三氟乙烯的现成可用度以及硼酸烯丙酯的丰富化学性质,使我们的方法适用于生物活性化合物的多种改性。初步的机理研究表明,α-氯硼酸酯是通过自由基过程活化的。
更新日期:2020-07-02
中文翻译:

通过还原镍催化,从乙烯基三氟甲磺酸酯和α-氯硼酸酯中得到烯丙基硼酸酯。
烯丙基硼酸酯是广泛用于有机合成的独特构建基,但是环状烯丙基硼酸酯的构建仍然是一个具有挑战性的课题。我们在这里展示了通过三氟甲磺酸酯和α-氯硼酸酯的交叉亲电子偶联,温和而有效地访问此类化合物的方法。反应以良好的底物范围和良好的官能团相容性进行。酮中三氟乙烯的现成可用度以及硼酸烯丙酯的丰富化学性质,使我们的方法适用于生物活性化合物的多种改性。初步的机理研究表明,α-氯硼酸酯是通过自由基过程活化的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号