当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Atomic Layer Etching of Gallium Oxide Using Sequential Exposures of HF and Various Metal Precursors
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.chemmater.0c00131 Younghee Lee 1 , Nicholas R. Johnson 1 , Steven M. George 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-06-19 , DOI: 10.1021/acs.chemmater.0c00131 Younghee Lee 1 , Nicholas R. Johnson 1 , Steven M. George 1
Affiliation
|
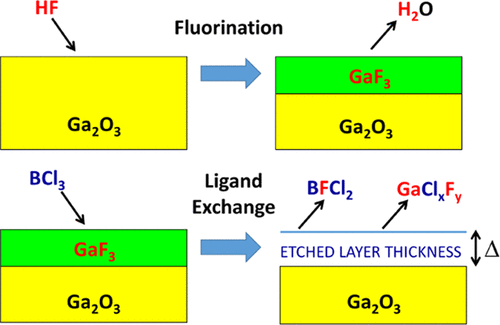
Gallium oxide (Ga2O3) is a transparent semiconducting oxide with a large band gap that has applications for power electronics and optoelectronics. Ga2O3 device fabrication requires etching for many processing steps. In this work, the thermal atomic layer etching (ALE) of Ga2O3 was performed using hydrofluoric acid (HF) and a wide range of different metal precursors including BCl3, AlCl(CH3)2, Al(CH3)3, TiCl4, and Ga(N(CH3)2)3. Because Ga2O3 is not a particularly stable oxide, the B-, Al-, or Ti-containing metal precursors can possibly convert the surface of Ga2O3 to B2O3, Al2O3, or TiO2. These metal precursors can also provide Cl, CH3, and N(CH3)2 ligands for ligand-exchange reactions. Consequently, the thermal ALE of Ga2O3 can occur via “conversion-etch” or fluorination and ligand-exchange reaction pathways. Using sequential HF and BCl3 exposures and in situ spectroscopic ellipsometry techniques, Ga2O3 etch rates were observed to vary from 0.59 to 1.35 Å/cycle at temperatures from 150 to 200 °C, respectively. The Ga2O3 etch rates were also self-limiting versus HF and BCl3 exposure. The lack of BCl3 pressure dependence for the etch rates argued against the conversion-etch mechanism and in favor of a fluorination and ligand-exchange reaction pathway. In situ quartz crystal microbalance techniques also revealed that Ga2O3 could be etched using sequential exposures of HF and various other metal precursors. Ga2O3 etch rates at 250 °C were 1.2, 0.82, 0.85, and 0.23 Å/cycle for AlCl(CH3)2, Al(CH3)3, TiCl4, and Ga(N(CH3)2)3 as the metal precursors, respectively. The mass changes during the individual exposures of HF and the AlCl(CH3)2 and Al(CH3)3 metal precursors argued for a fluorination and ligand-exchange mechanism. The AlCl(CH3)2 and Al(CH3)3 exposures may also lead to some conversion of Ga2O3 to Al2O3. In contrast, the mass changes during the HF and TiCl4 exposures were consistent with the conversion of the surface of Ga2O3 to TiO2 and then the spontaneous removal of the TiO2 surface layer by HF. Distinctly different behavior was observed during the HF and Ga(N(CH3)2)3 exposures. The large mass gain during the Ga(N(CH3)2)3 exposures suggested that Ga(N(CH3)2)3 can adsorb on the fluorinated Ga2O3 surface prior to the ligand-exchange reaction. The wide range of metal precursors that can etch Ga2O3 argues that the ability of these precursors to convert Ga2O3 or to undergo ligand-exchange reactions provides multiple pathways for effective thermal Ga2O3 ALE.
中文翻译:

使用HF和各种金属前体的顺序曝光对氧化镓进行热原子层蚀刻
氧化镓(Ga 2 O 3)是一种具有大带隙的透明半导体氧化物,已在电力电子和光电子学中得到应用。Ga 2 O 3器件的制造需要许多工艺步骤的蚀刻。在这项工作中,使用氢氟酸(HF)和包括BCl 3,AlCl(CH 3)2,Al(CH 3)3在内的各种不同的金属前体对Ga 2 O 3进行热原子层蚀刻(ALE)。,TiCl 4和Ga(N(CH 3)2)3。因为Ga 2O 3不是特别稳定的氧化物,含B,Al或Ti的金属前体可能会将Ga 2 O 3的表面转化为B 2 O 3,Al 2 O 3或TiO 2。这些金属前体还可为配体交换反应提供Cl,CH 3和N(CH 3)2配体。因此,Ga 2 O 3的热ALE可以通过“转化蚀刻”或氟化和配体交换反应途径发生。使用连续的HF和BCl 3曝光以及原位光谱椭圆仪技术,Ga在150至200°C的温度下,分别观察到2 O 3蚀刻速率在0.59至1.35Å/循环之间变化。Ga 2 O 3蚀刻速率相对于HF和BCl 3暴露也是自限的。缺乏BCl 3对蚀刻速率的压力依赖性,这与转化-蚀刻机理相反,并且有利于氟化和配体交换反应途径。原位石英晶体微天平技术还显示,可以使用HF和各种其他金属前体的顺序曝光来蚀刻Ga 2 O 3。对于AlCl(CH),Ga 2 O 3在250°C的蚀刻速率为1.2、0.82、0.85和0.23Å/循环3)2,Al(CH 3)3,TiCl 4和Ga(N(CH 3)2)3分别作为金属前体。在HF和AlCl(CH 3)2和Al(CH 3)3金属前体的单独暴露过程中,质量变化表明存在氟化和配体交换机理。AlCl(CH 3)2和Al(CH 3)3暴露也可能导致Ga 2 O 3转化为Al 2 O 3。相反,在HF和TiCl 4暴露期间的质量变化与Ga 2 O 3的表面向TiO 2的转化以及然后通过HF自发地去除TiO 2表面层一致。在HF和Ga(N(CH 3)2)3暴露期间观察到明显不同的行为。Ga(N(CH 3)2)3暴露期间的大质量增加表明Ga(N(CH 3)2)3可以吸附在氟化Ga 2 O 3上在配体交换反应之前的表面。可以蚀刻Ga 2 O 3的各种各样的金属前驱物认为,这些前驱物转化Ga 2 O 3或进行配体交换反应的能力为有效的热Ga 2 O 3 ALE提供了多种途径。
更新日期:2020-07-28
中文翻译:

使用HF和各种金属前体的顺序曝光对氧化镓进行热原子层蚀刻
氧化镓(Ga 2 O 3)是一种具有大带隙的透明半导体氧化物,已在电力电子和光电子学中得到应用。Ga 2 O 3器件的制造需要许多工艺步骤的蚀刻。在这项工作中,使用氢氟酸(HF)和包括BCl 3,AlCl(CH 3)2,Al(CH 3)3在内的各种不同的金属前体对Ga 2 O 3进行热原子层蚀刻(ALE)。,TiCl 4和Ga(N(CH 3)2)3。因为Ga 2O 3不是特别稳定的氧化物,含B,Al或Ti的金属前体可能会将Ga 2 O 3的表面转化为B 2 O 3,Al 2 O 3或TiO 2。这些金属前体还可为配体交换反应提供Cl,CH 3和N(CH 3)2配体。因此,Ga 2 O 3的热ALE可以通过“转化蚀刻”或氟化和配体交换反应途径发生。使用连续的HF和BCl 3曝光以及原位光谱椭圆仪技术,Ga在150至200°C的温度下,分别观察到2 O 3蚀刻速率在0.59至1.35Å/循环之间变化。Ga 2 O 3蚀刻速率相对于HF和BCl 3暴露也是自限的。缺乏BCl 3对蚀刻速率的压力依赖性,这与转化-蚀刻机理相反,并且有利于氟化和配体交换反应途径。原位石英晶体微天平技术还显示,可以使用HF和各种其他金属前体的顺序曝光来蚀刻Ga 2 O 3。对于AlCl(CH),Ga 2 O 3在250°C的蚀刻速率为1.2、0.82、0.85和0.23Å/循环3)2,Al(CH 3)3,TiCl 4和Ga(N(CH 3)2)3分别作为金属前体。在HF和AlCl(CH 3)2和Al(CH 3)3金属前体的单独暴露过程中,质量变化表明存在氟化和配体交换机理。AlCl(CH 3)2和Al(CH 3)3暴露也可能导致Ga 2 O 3转化为Al 2 O 3。相反,在HF和TiCl 4暴露期间的质量变化与Ga 2 O 3的表面向TiO 2的转化以及然后通过HF自发地去除TiO 2表面层一致。在HF和Ga(N(CH 3)2)3暴露期间观察到明显不同的行为。Ga(N(CH 3)2)3暴露期间的大质量增加表明Ga(N(CH 3)2)3可以吸附在氟化Ga 2 O 3上在配体交换反应之前的表面。可以蚀刻Ga 2 O 3的各种各样的金属前驱物认为,这些前驱物转化Ga 2 O 3或进行配体交换反应的能力为有效的热Ga 2 O 3 ALE提供了多种途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号