当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipase-Catalyzed Ring-Opening Polymerization of Benzyl Malolactonate: An Unusual Mechanism?
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.biomac.0c00593 Hubert Casajus 1 , Eric Dubreucq 2 , Sylvain Tranchimand 1 , Véronique Perrier 2 , Caroline Nugier-Chauvin 1 , Sandrine Cammas-Marion 1, 3
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.biomac.0c00593 Hubert Casajus 1 , Eric Dubreucq 2 , Sylvain Tranchimand 1 , Véronique Perrier 2 , Caroline Nugier-Chauvin 1 , Sandrine Cammas-Marion 1, 3
Affiliation
|
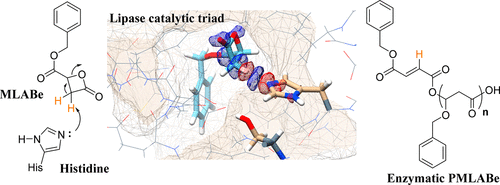
The use of safe natural catalyst such as enzymes for ring opening polymerization (ROP) of β-substituted β-lactones such as benzyl malolactonate (MLABe) is an important objective considering the biomedical applications of the resulting (co)polymers. However, the preparation of well-defined polymeric materials using such systems requires an understanding of enzyme–substrate interactions. In this context, we investigated the mechanism of lipase-catalyzed ROP of MLABe, because it appears that it is probably not the same as the one widely described for other lactones such ε-caprolactone, propiolactone. and lactide. Enzymatic-catalyzed ROPs of MLABe in the presence of the lipase/acyltransferase CpLip2 and its serine knockout (serine KO) mutant (CpLip2_180A) have led to poly(benzyl malate) (PMLABe) terminated by a monobenzyl fumarate group with monomer conversion higher than 70% and weight-average molar mass of about 3600 g/mol (Đ = 1.42). On the other hand, only less than 7% of MLABe conversion and no polymer formation were observed when the polymerization reaction was conducted in the presence of inactivated CpLip2 (heated at 100 °C). Moreover, the ROP of MLABe in the presence of imidazole, a synthetic mimic of the catalytic histidine, led to a PMLABe terminated by a monobenzyl fumarate group. On the contrary, neither the enzymatic-catalyzed ROP of benzyl dimethylmalolactonate (diMeMLABe), a MLABe with two methyl groups instead of the two “acidic” protons on the lactone’s ring, in the presence of CpLip2 and CpLip2_180A nor its chemical ROP in the presence of imidazole were successful. Together, all these results suggested that the lipase-catalyzed polymerization of malolactonates occurred through the abstraction of one of the two “acidic” protons of the lactone’s ring by the histidine of the catalytic triad leading to the corresponding monobenzyl fumarate responsible for the polymerization of the remaining monomer. Finally, molecular modeling of the positioning of the monomer into the catalytic site of the CpLip2 and DFT quantum-chemical calculations highlighted an interaction of (R)- and (S)-MLABe with the catalytic histidine of the enzyme preferentially to serine, in the form of a strong hydrogen bond with one of the “acidic” protons of MLABe, thus, supporting the important role of the catalytic histidine in the polymerization of such cyclic lactones.
中文翻译:

脂肪酶催化苹果酸丙二酸苄酯的开环聚合:一个不常见的机制?
考虑到所得(共)聚合物的生物医学应用,使用安全的天然催化剂,例如酶用于β-取代的β-内酯的开环聚合(ROP),例如苹果酸内酯(MLABe)。但是,使用此类系统制备定义明确的聚合物材料需要了解酶与底物之间的相互作用。在这种情况下,我们研究了脂肪酶催化的MLABe ROP的机理,因为它似乎与广泛描述的其他内酯(如ε-己内酯,丙内酯)不同。和丙交酯。Đ= 1.42)。另一方面,当聚合反应在失活的CpLip2(加热到100°C)的情况下进行时,仅观察到不到7%的MLABe转化率,没有观察到聚合物的形成。此外,在咪唑(催化组氨酸的合成模拟物)存在下,MLABe的ROP导致被富马酸单苄基酯封端的PMLABe。相反,在存在CpLip2和CpLip2_180A的情况下,既不存在内酯环上具有两个甲基而不是两个“酸性”质子的MLABe也不是酶催化的苄基二甲基丙二酸苄基二甲酯(diMeMLABe)。咪唑治疗成功。一起,所有这些结果表明,丙三酸内酯的脂肪酶催化的聚合反应是通过催化三单元组的组氨酸抽象化内酯环的两个“酸性”质子之一而导致的,相应的富马酸单苄酯负责剩余单体的聚合反应。 。最后,将单体定位到CpLip2催化位点的分子模型和DFT量子化学计算突显了(R)和(S)-MLABe与酶的催化组氨酸优先于丝氨酸,呈强氢键形式与MLABe的“酸性”质子之一结合,从而支持了催化组氨酸在这种环状内酯的聚合。
更新日期:2020-07-13
中文翻译:

脂肪酶催化苹果酸丙二酸苄酯的开环聚合:一个不常见的机制?
考虑到所得(共)聚合物的生物医学应用,使用安全的天然催化剂,例如酶用于β-取代的β-内酯的开环聚合(ROP),例如苹果酸内酯(MLABe)。但是,使用此类系统制备定义明确的聚合物材料需要了解酶与底物之间的相互作用。在这种情况下,我们研究了脂肪酶催化的MLABe ROP的机理,因为它似乎与广泛描述的其他内酯(如ε-己内酯,丙内酯)不同。和丙交酯。Đ= 1.42)。另一方面,当聚合反应在失活的CpLip2(加热到100°C)的情况下进行时,仅观察到不到7%的MLABe转化率,没有观察到聚合物的形成。此外,在咪唑(催化组氨酸的合成模拟物)存在下,MLABe的ROP导致被富马酸单苄基酯封端的PMLABe。相反,在存在CpLip2和CpLip2_180A的情况下,既不存在内酯环上具有两个甲基而不是两个“酸性”质子的MLABe也不是酶催化的苄基二甲基丙二酸苄基二甲酯(diMeMLABe)。咪唑治疗成功。一起,所有这些结果表明,丙三酸内酯的脂肪酶催化的聚合反应是通过催化三单元组的组氨酸抽象化内酯环的两个“酸性”质子之一而导致的,相应的富马酸单苄酯负责剩余单体的聚合反应。 。最后,将单体定位到CpLip2催化位点的分子模型和DFT量子化学计算突显了(R)和(S)-MLABe与酶的催化组氨酸优先于丝氨酸,呈强氢键形式与MLABe的“酸性”质子之一结合,从而支持了催化组氨酸在这种环状内酯的聚合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号