当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Diversity of Native Major Ampullate, Minor Ampullate, Cylindriform, and Flagelliform Silk Proteins in Solution.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.biomac.0c00819 Imke Greving 1 , Ann E Terry 2, 3 , Chris Holland 4 , Maxime Boulet-Audet 5 , Isabelle Grillo 6 , Fritz Vollrath 7 , Cedric Dicko 3, 8
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.biomac.0c00819 Imke Greving 1 , Ann E Terry 2, 3 , Chris Holland 4 , Maxime Boulet-Audet 5 , Isabelle Grillo 6 , Fritz Vollrath 7 , Cedric Dicko 3, 8
Affiliation
|
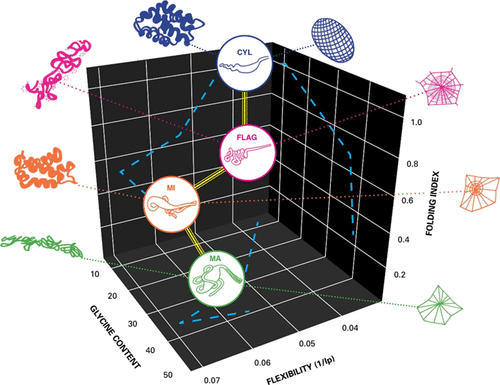
The foundations of silk spinning, the structure, storage, and activation of silk proteins, remain highly debated. By combining solution small-angle neutron and X-ray scattering (SANS and SAXS) alongside circular dichroism (CD), we reveal a shape anisotropy of the four principal native spider silk feedstocks from Nephila edulis. We show that these proteins behave in solution like elongated semiflexible polymers with locally rigid sections. We demonstrated that minor ampullate and cylindriform proteins adopt a monomeric conformation, while major ampullate and flagelliform proteins have a preference for dimerization. From an evolutionary perspective, we propose that such dimerization arose to help the processing of disordered silk proteins. Collectively, our results provide insights into the molecular-scale processing of silk, uncovering a degree of evolutionary convergence in protein structures and chemistry that supports the macroscale micellar/pseudo liquid crystalline spinning mechanisms proposed by the community.
中文翻译:

溶液中天然大壶腹、小壶腹、圆柱状和鞭毛状丝蛋白的结构多样性。
丝纺的基础、丝蛋白的结构、储存和活化仍然存在激烈争论。通过将溶液小角中子和 X 射线散射(SANS 和 SAXS)与圆二色性 (CD) 相结合,我们揭示了来自喜鹊的四种主要天然蜘蛛丝原料的形状各向异性。我们证明这些蛋白质在溶液中的行为类似于具有局部刚性部分的细长半柔性聚合物。我们证明,小壶腹和圆柱状蛋白质采用单体构象,而大壶腹和鞭毛状蛋白质则倾向于二聚化。从进化的角度来看,我们认为这种二聚化的出现有助于无序丝蛋白的加工。总的来说,我们的结果提供了对丝的分子尺度加工的见解,揭示了蛋白质结构和化学的一定程度的进化趋同,支持了社区提出的宏观胶束/伪液晶纺丝机制。
更新日期:2020-08-10
中文翻译:

溶液中天然大壶腹、小壶腹、圆柱状和鞭毛状丝蛋白的结构多样性。
丝纺的基础、丝蛋白的结构、储存和活化仍然存在激烈争论。通过将溶液小角中子和 X 射线散射(SANS 和 SAXS)与圆二色性 (CD) 相结合,我们揭示了来自喜鹊的四种主要天然蜘蛛丝原料的形状各向异性。我们证明这些蛋白质在溶液中的行为类似于具有局部刚性部分的细长半柔性聚合物。我们证明,小壶腹和圆柱状蛋白质采用单体构象,而大壶腹和鞭毛状蛋白质则倾向于二聚化。从进化的角度来看,我们认为这种二聚化的出现有助于无序丝蛋白的加工。总的来说,我们的结果提供了对丝的分子尺度加工的见解,揭示了蛋白质结构和化学的一定程度的进化趋同,支持了社区提出的宏观胶束/伪液晶纺丝机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号