当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potentiometric Titration Method for the Determination of Solubility Limits and pKa Values of Weak Organic Acids in Water.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.analchem.0c00247 Eduard Wiedenbeck 1 , Denis Gebauer 1 , Helmut Cölfen 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.analchem.0c00247 Eduard Wiedenbeck 1 , Denis Gebauer 1 , Helmut Cölfen 1
Affiliation
|
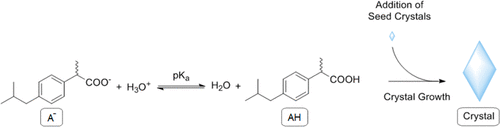
The determination of solubility limits of compounds in water is unprecise and relies on certain prerequisites such as UV–vis absorption activity. In this study, we designed an experimental approach based on potentiometric titrations to determine solubility limits of various organic compounds by exploiting their pH-active carboxylic acid groups. By applying the law of mass action, utilizing a double-dosing method ensuring a constant compound concentration, it is possible to determine the intrinsic solubility limits, which are independent of the pH value. The derived equations enable the precise and fast determination of intrinsic solubility limits of organic compounds in aqueous solutions within 2–4 h. Moreover, it is shown how the pKa value can be determined based on titrations carried out at two different compound concentrations.
中文翻译:

电位滴定法测定水中弱有机酸的溶解度限值和pKa值。
化合物在水中的溶解度极限的测定是不精确的,并且取决于某些先决条件,例如UV-vis吸收活性。在这项研究中,我们设计了一种基于电位滴定的实验方法,通过利用各种有机化合物的pH活性羧酸基来确定其溶解度极限。通过应用质量作用定律,采用确保化合物浓度恒定的双剂量方法,可以确定固有溶解度极限,该极限与pH值无关。导出的方程式可以在2-4小时内精确,快速地确定有机化合物在水溶液中的固有溶解度极限。此外,还显示了p K a 可以基于在两种不同化合物浓度下进行的滴定确定值。
更新日期:2020-07-21
中文翻译:

电位滴定法测定水中弱有机酸的溶解度限值和pKa值。
化合物在水中的溶解度极限的测定是不精确的,并且取决于某些先决条件,例如UV-vis吸收活性。在这项研究中,我们设计了一种基于电位滴定的实验方法,通过利用各种有机化合物的pH活性羧酸基来确定其溶解度极限。通过应用质量作用定律,采用确保化合物浓度恒定的双剂量方法,可以确定固有溶解度极限,该极限与pH值无关。导出的方程式可以在2-4小时内精确,快速地确定有机化合物在水溶液中的固有溶解度极限。此外,还显示了p K a 可以基于在两种不同化合物浓度下进行的滴定确定值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号