当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Residues of helix ɑ2 are critical for catalytic efficiency of mycobacterial alkylhydroperoxide reductase subunit C
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-20 , DOI: 10.1002/1873-3468.13864 Shi Min Sherilyn Chong 1, 2, 3 , Neelagandan Kamariah 1 , Gerhard Grüber 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-20 , DOI: 10.1002/1873-3468.13864 Shi Min Sherilyn Chong 1, 2, 3 , Neelagandan Kamariah 1 , Gerhard Grüber 1
Affiliation
|
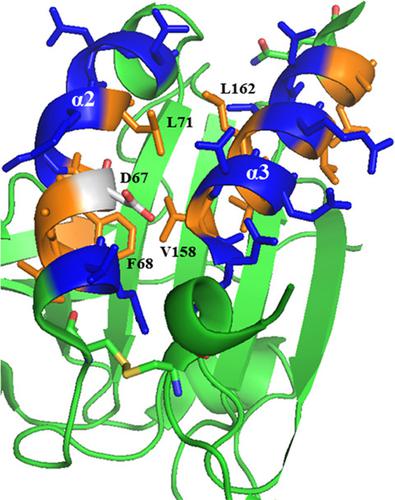
The ability of Mycobacteria to overcome oxidative stress is of paramount importance for its survival within the host. One of the key enzymes that are involved in protecting the bacterium from reactive oxygen species is the catalase‐peroxidase (KatG). However, in strains resistant to the antibiotic isoniazid, KatG is rendered ineffective, which is associated with an increased expression of alkylhydroperoxide reductase subunit C (AhpC). Mycobacterial AhpC possesses a unique helical displacement when compared to its bacterial counterparts. Here, via mutagenesis studies, we demonstrate the importance of this helix for redox modulation of the catalytic activity of AhpC. Along with structural insights from crystallographic data, the impact of critical residues on the structure and flexibility of the helix and on AhpC oligomerization is described.
中文翻译:

螺旋 ɑ2 的残基对分枝杆菌烷基氢过氧化物还原酶亚基 C 的催化效率至关重要
分枝杆菌克服氧化应激的能力对其在宿主内的生存至关重要。过氧化氢酶-过氧化物酶 (KatG) 是保护细菌免受活性氧侵害的关键酶之一。然而,在对抗生素异烟肼具有抗性的菌株中,KatG 变得无效,这与烷基氢过氧化物还原酶亚基 C (AhpC) 的表达增加有关。与细菌对应物相比,分枝杆菌 AhpC 具有独特的螺旋位移。在这里,通过诱变研究,我们证明了该螺旋对于 AhpC 催化活性的氧化还原调节的重要性。除了晶体学数据的结构见解外,还描述了关键残基对螺旋结构和灵活性以及 AhpC 寡聚化的影响。
更新日期:2020-07-20
中文翻译:

螺旋 ɑ2 的残基对分枝杆菌烷基氢过氧化物还原酶亚基 C 的催化效率至关重要
分枝杆菌克服氧化应激的能力对其在宿主内的生存至关重要。过氧化氢酶-过氧化物酶 (KatG) 是保护细菌免受活性氧侵害的关键酶之一。然而,在对抗生素异烟肼具有抗性的菌株中,KatG 变得无效,这与烷基氢过氧化物还原酶亚基 C (AhpC) 的表达增加有关。与细菌对应物相比,分枝杆菌 AhpC 具有独特的螺旋位移。在这里,通过诱变研究,我们证明了该螺旋对于 AhpC 催化活性的氧化还原调节的重要性。除了晶体学数据的结构见解外,还描述了关键残基对螺旋结构和灵活性以及 AhpC 寡聚化的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号