Tetrahedron ( IF 2.1 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.tet.2020.131340 Anastasia A. Fesenko , Anatoly D. Shutalev
|
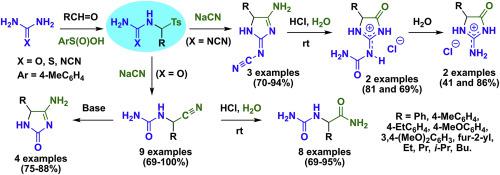
Reaction of N-(tosylmethyl)-substituted ureas, thioureas, and N′-cyanoguanidines, prepared by condensation of the corresponding amides with various aldehydes and p-toluenesulfinic acid, with NaCN has been studied. The outcome of the reaction is strongly dependent on the amide nature and reaction conditions. Generally, N-(tosylmethyl)ureas afford α-ureido nitriles, N-(tosylmethyl)-N′-cyanoguanidines transform into 4-amino-2-cyanimino-1,5-dihydro-2H-imidazoles, and N-(tosylmethyl)thioureas give complex mixtures of various imidazole derivatives. The prepared α-ureido nitriles are selectively converted into the corresponding α-ureido amides by treatment with conc. HCl at room temperature. Under basic conditions, α-ureido nitriles cyclize into 4-amino-1,5-dihydro-2H-imidazole-2-ones. Treatment of 4-amino-2-cyanimino-1,5-dihydro-2H-imidazoles with conc. HCl at room temperature lead to hydrolysis of both the amidine fragment and cyano group to give hydrochlorides of 2-(carbamoylimino)imidazolidin-4-ones which are easily decarbamoylated to form hydrochlorides of 2-iminoimidazolidin-4-ones. Structure of the prepared imidazoles and some mechanistic aspects of cyanide-anion amidoalkylation with N-(tosylmethyl)ureas are discussed based on DFT calculations.
中文翻译:

N-(甲苯磺酰基甲基)取代的脲,硫脲和N'-氰基胍与氰化钠反应的不同途径。α-脲基腈,α-脲基酰胺和乙内酰脲亚氨基衍生物的合成
研究了通过相应的酰胺与各种醛和对甲苯亚磺酸缩合制备的N-(甲苯磺酰基甲基)取代的脲,硫脲和N'-氰基胍与NaCN的反应。反应的结果在很大程度上取决于酰胺的性质和反应条件。通常,Ñ - (甲苯磺酰基甲基)脲,得到α -脲基腈,ñ - (甲苯磺酰基甲基) - N' -cyanoguanidines转变成4-氨基-2-氰基亚-1,5-二氢-2 ħ -咪唑,和Ñ-(甲苯磺酰基甲基)硫脲得到各种咪唑衍生物的复杂混合物。通过用浓溶液处理将制备的α-脲基腈选择性地转化为相应的α-脲基酰胺。在室温下为HCl。在碱性条件下,α-脲基腈环化成4-氨基-1,5-二氢-2 H-咪唑-2-酮。用浓溶液处理4-氨基-2-氰基亚氨基-1,5-二氢-2 H-咪唑。室温下的HCl导致hydrolysis片段和氰基水解,得到2-(氨基甲酰亚氨基)咪唑啉-4-酮的盐酸盐,其易于被氨基甲酰化而形成2-亚氨基咪唑啉-4-酮的盐酸盐。制备的咪唑的结构以及N与氰化物-阴离子酰胺烷基化反应的一些机理基于DFT计算讨论了-(甲苯磺酰基甲基)脲。











































 京公网安备 11010802027423号
京公网安备 11010802027423号