Journal of Structural Biology ( IF 3 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.jsb.2020.107552 Deepak Pathak 1 , Eunju Kwon 1 , Dong Young Kim 1
|
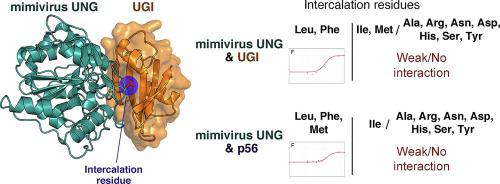
Uracil-N-glycosylase (UNG) is found in most organisms as well as in large DNA viruses. Its inhibitory proteins, including uracil glycosylase inhibitor (UGI) and p56, tightly bind to the active site of UNG by mimicking the DNA substrates. As the binding motifs are conserved in UNG family proteins, the inhibitory proteins bind to various UNG proteins across species. However, the intercalation residue that penetrates the DNA minor groove during uracil excision is not conserved among UNG proteins. To understand the role of the intercalation residue in their binding to the inhibitory proteins, we prepared mutants of mimivirus UNG, measured the binding affinity between the UNG mutants and inhibitory proteins, and analyzed the interactions based on the crystal structures of mimivirus UNG mutants complexed with UGI. The results show that mimivirus UNG, which harbors Tyr as an intercalation residue, did not interact with the inhibitory proteins intrinsically, whereas mutations of the intercalation residue to Phe or Leu resulted in tight interactions with UGI and p56; mutation to Met resulted in tight interactions only with p56. The crystal structures revealed that Phe and Leu stabilize the interactions by fitting into the hydrophobic pocket of UGI. These results show that differences in size and hydrophobicity of the intercalation residues determine the interactions between UNG family proteins and the inhibitory proteins, UGI and p56.
中文翻译:

模拟病毒尿嘧啶-DNA 糖基化酶和由单个氨基酸决定的抑制蛋白之间的选择性相互作用。
尿嘧啶-N-糖基化酶 (UNG) 存在于大多数生物体以及大型 DNA 病毒中。其抑制蛋白,包括尿嘧啶糖基化酶抑制剂 (UGI) 和 p56,通过模拟 DNA 底物与 UNG 的活性位点紧密结合。由于结合基序在 UNG 家族蛋白中是保守的,因此抑制性蛋白与不同物种的各种 UNG 蛋白结合。然而,在尿嘧啶切除过程中穿透 DNA 小沟的嵌入残基在 UNG 蛋白中并不保守。为了了解嵌入残基在与抑制蛋白结合中的作用,我们制备了拟杆菌 UNG 的突变体,测量了 UNG 突变体与抑制蛋白之间的结合亲和力,并基于与地理标志。结果表明,拟菌病毒 UNG,含有 Tyr 作为嵌入残基的蛋白,本质上不与抑制蛋白相互作用,而嵌入残基突变为 Phe 或 Leu 导致与 UGI 和 p56 的紧密相互作用;Met 突变导致仅与 p56 紧密相互作用。晶体结构表明,Phe 和 Leu 通过嵌入 UGI 的疏水口袋来稳定相互作用。这些结果表明,嵌入残基的大小和疏水性差异决定了 UNG 家族蛋白与抑制蛋白 UGI 和 p56 之间的相互作用。Met 突变导致仅与 p56 紧密相互作用。晶体结构表明,Phe 和 Leu 通过嵌入 UGI 的疏水口袋来稳定相互作用。这些结果表明,嵌入残基的大小和疏水性差异决定了 UNG 家族蛋白与抑制蛋白 UGI 和 p56 之间的相互作用。Met 突变导致仅与 p56 紧密相互作用。晶体结构表明,Phe 和 Leu 通过嵌入 UGI 的疏水口袋来稳定相互作用。这些结果表明,嵌入残基的大小和疏水性差异决定了 UNG 家族蛋白与抑制蛋白 UGI 和 p56 之间的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号