Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.jmb.2020.06.015 Michael Reidy 1 , Daniel C Masison 1
|
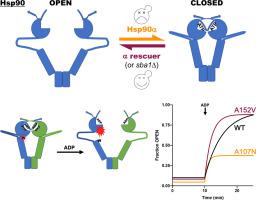
Hsp90 is a highly conserved molecular chaperone important for the activity of many client proteins. Hsp90 has an N-terminal ATPase domain (N), a middle domain (M) that interacts with clients and a C-terminal dimerization domain (C). “Closing” of dimers around clients is regulated by ATP binding, co-chaperones, and post-translational modifications. ATP hydrolysis coincides with release of mature client and resetting the reaction cycle. Humans have two Hsp90s: hHsp90α and hHsp90β. Although 85% identical, hHsp90β supports Hsp90 function in yeast much better than hHsp90α. Determining the basis of this difference would provide important insight into functional specificity of seemingly redundant Hsp90s, and the evolution of eukaryotic Hsp90 systems and clientele. Here, we found host co-chaperones Sba1, Cpr6 and Cpr7 inhibited hHsp90α function in yeast, and we identified mutations clustering in the N domain that considerably improved hHsp90α function in yeast. The strongest of these rescuer mutations accelerated nucleotide-dependent lid closing, N–M domain docking, and ATPase. It also disrupted binding to Sba1, which prolongs the closed state, and promoted N–M undocking and lid opening. Our data suggest the rescuer mutations improve function of hHsp90α in yeast by accelerating return to the open state. Our findings imply hHsp90α occupies the closed state too long to function effectively in yeast, and define an evolutionarily conserved region of the N domain involved in resetting the Hsp90 reaction cycle.
中文翻译:

Hsp90 N 结构域中的突变可识别酵母中控制二聚体打开并扩展人类 Hsp90α 功能的位点。
Hsp90 是一种高度保守的分子伴侣,对于许多客户蛋白的活性非常重要。 Hsp90 具有 N 端 ATP 酶结构域 (N)、与客户端相互作用的中间结构域 (M) 和 C 端二聚化结构域 (C)。客户周围二聚体的“闭合”受到 ATP 结合、共伴侣和翻译后修饰的调节。 ATP 水解与成熟客户的释放和重置反应周期同时发生。人类有两个 Hsp90:hHsp90α 和 hHsp90β。尽管 85% 相同,hHsp90β 比 hHsp90α 更好地支持酵母中的 Hsp90 功能。确定这种差异的基础将为了解看似冗余的 Hsp90 的功能特异性以及真核 Hsp90 系统和客户的进化提供重要的见解。在这里,我们发现宿主共伴侣 Sba1、Cpr6 和 Cpr7 抑制酵母中的 hHsp90α 功能,并且我们发现 N 结构域中聚集的突变显着改善了酵母中的 hHsp90α 功能。这些救援突变中最强的一个加速了核苷酸依赖性盖子关闭、N-M 结构域对接和 ATP 酶。它还破坏了与 Sba1 的结合,从而延长了关闭状态,并促进了 N-M 脱扣和盖子打开。我们的数据表明,救援突变通过加速返回开放状态来改善酵母中 hHsp90α 的功能。我们的研究结果表明,hHsp90α 处于封闭状态的时间太长,无法在酵母中有效发挥作用,并定义了参与重置 Hsp90 反应周期的 N 结构域的进化保守区域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号