Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.bioorg.2020.104029 Mengting Wang 1 , Jianchu Chen 1 , Xingqian Ye 1 , Donghong Liu 2
|
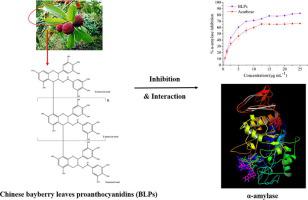
Chinese bayberry leaves proanthocyanidins (BLPs) belongs to the prodelphinidin category with potent EGCG unit, whose inhibition effect on α-amylase and their interaction were investigated by in vitro digestion and enzyme kinetic analysis, multi fluorescence spectroscopies (fluorescence quenching, synchronous fluorescence, and three-dimensional fluorescence), circular dichroism spectra, Fourier transform infrared spectroscopy and in silico modelling. The results revealed that BLPs was a mixed inhibitor to α-amylase with the IC50 value of 3.075 ± 0.073 μg/mL. BLPs could lead to a static fluorescence quenching of α-amylase, mainly by means of interacting with amino acids (mainly Try and Tyr residues) in one site on α-amylase molecule under the action of hydrogen bonding and/or Van der Waals force. This interaction further induced the change of secondary conformational structure, functional group structure and hydrophobicity of α-amylase, thus resulting in lowering activity. Molecular docking simulated that this binding occurred in a cavity on the surface of the α-amylase molecule, and BLPs trimer showed a relatively high binding energy. The present study provided a new insight of BLPs as an α-amylase inhibitor, which could be considered in anti-diabetic therapy.
中文翻译:

杨梅(Myrica rubra Sieb。et Zucc。)的体外抑制作用原花青素对胰腺α-淀粉酶及其相互作用具有抑制作用。
杨梅叶原花青素(BLPs)属于具有强EGCG单元的原花青素类,通过体外消化和酶动力学分析,多荧光光谱(荧光猝灭,同步荧光和三种)研究了其对α-淀粉酶的抑制作用及其相互作用。维荧光),圆二色性光谱,傅立叶变换红外光谱和计算机模拟。结果表明,BLP与IC 50混合使用是α-淀粉酶的混合抑制剂。值为3.075±0.073μg/ mL。BLP可以通过在氢键和/或范德华力的作用下与α-淀粉酶分子上一个位点的氨基酸(主要是Try和Tyr残基)相互作用来导致α-淀粉酶的静态荧光猝灭。这种相互作用进一步引起α-淀粉酶的二级构象结构,官能团结构和疏水性的改变,从而导致活性降低。分子对接模拟表明这种结合发生在α-淀粉酶分子表面的空腔中,而BLP三聚体显示出较高的结合能。本研究为BLP作为α-淀粉酶抑制剂提供了新的见解,可以在抗糖尿病治疗中加以考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号