当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silver(I)-catalyzed selective hydroalkoxylation of C2-alkynyl quinazolinones to synthesize quinazolinone-fused eight-membered N,O-heterocycles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0qo00437e Xiang-Fei Kong 1, 2, 3, 4, 5 , Xiu-Yun Guo 1, 2, 3, 4, 5 , Zi-Yu Gu 1, 2, 3, 4, 5 , Lin-Su Wei 1, 2, 3, 4, 5 , Lu-Lu Liu 1, 2, 3, 4, 5 , Dong-Liang Mo 1, 2, 3, 4, 5 , Cheng-Xue Pan 1, 2, 3, 4, 5 , Gui-Fa Su 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0qo00437e Xiang-Fei Kong 1, 2, 3, 4, 5 , Xiu-Yun Guo 1, 2, 3, 4, 5 , Zi-Yu Gu 1, 2, 3, 4, 5 , Lin-Su Wei 1, 2, 3, 4, 5 , Lu-Lu Liu 1, 2, 3, 4, 5 , Dong-Liang Mo 1, 2, 3, 4, 5 , Cheng-Xue Pan 1, 2, 3, 4, 5 , Gui-Fa Su 1, 2, 3, 4, 5
Affiliation
|
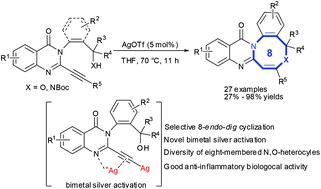
We report a silver-catalyzed hydroalkoxylation of C2-alkynyl quinazolinones to prepare a series of novel quinazolinone-fused eight-membered N,O-heterocycles in good-to-excellent yields through a selective 8-endo-dig cyclization. Mechanistic studies revealed that the silver catalyst might aid bidentate coordination of an imine group and alkyne to facilitate 8-endo-dig cyclization to afford eight-membered N,O-heterocycles. Also, the proposed bimetal silver intermediates might promote hydroalkoxylation rapidly for quinazolinones bearing terminal alkynes at the C2-position. Biological evaluations revealed that most of the designed quinazolinone-fused eight-membered N,O-heterocycles inhibited nitric-oxide generation significantly in lipopolysaccharide-stimulated RAW264.7 cells and displayed their bioactivity as potentially good anti-inflammatory agents.
中文翻译:

银(I)催化的C2-炔基喹唑啉酮的选择性加氢烷氧基化反应合成喹唑啉酮稠合的八元N,O-杂环
我们报告了银催化的C2-炔基喹唑啉酮的加氢烷氧基化反应,通过选择性的8-内-地-环化制备了一系列新颖的喹唑啉酮稠合的八元N,O-杂环,并具有良好至优异的产率。机理研究表明,银催化剂可能有助于亚胺基和炔烃的双齿配位,以促进8-内挖环化得到八元N,O-杂环。同样,对于在C 2位带有末端炔烃的喹唑啉酮,建议的双金属银中间体可能会迅速促进加氢烷氧基化。生物学评估表明,大多数设计的喹唑啉酮融合的八元N,O-杂环在脂多糖刺激的RAW264.7细胞中均能显着抑制一氧化氮的产生,并显示出其作为潜在的良好抗炎剂的生物活性。
更新日期:2020-07-28
中文翻译:

银(I)催化的C2-炔基喹唑啉酮的选择性加氢烷氧基化反应合成喹唑啉酮稠合的八元N,O-杂环
我们报告了银催化的C2-炔基喹唑啉酮的加氢烷氧基化反应,通过选择性的8-内-地-环化制备了一系列新颖的喹唑啉酮稠合的八元N,O-杂环,并具有良好至优异的产率。机理研究表明,银催化剂可能有助于亚胺基和炔烃的双齿配位,以促进8-内挖环化得到八元N,O-杂环。同样,对于在C 2位带有末端炔烃的喹唑啉酮,建议的双金属银中间体可能会迅速促进加氢烷氧基化。生物学评估表明,大多数设计的喹唑啉酮融合的八元N,O-杂环在脂多糖刺激的RAW264.7细胞中均能显着抑制一氧化氮的产生,并显示出其作为潜在的良好抗炎剂的生物活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号