当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling performance of rhamnolipid-coated engineered magnetite nanoparticles for U(VI) sorption and separation
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0en00416b Neha Sharma 1, 2, 3, 4 , Anushree Ghosh 1, 2, 3, 4 , John D. Fortner 4, 5, 6, 7 , Daniel E. Giammar 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0en00416b Neha Sharma 1, 2, 3, 4 , Anushree Ghosh 1, 2, 3, 4 , John D. Fortner 4, 5, 6, 7 , Daniel E. Giammar 1, 2, 3, 4
Affiliation
|
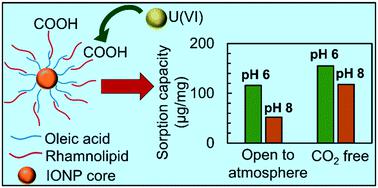
Based on tunable properties, engineered nanoparticles (NPs) hold significant promise for water treatment technologies. Motivated by concerns regarding toxicity and non-biodegradability of some nanoparticles, we explored engineered magnetite (Fe3O4) nanoparticles with a biocompatible coating. These were prepared with a coating of rhamnolipid, a biosurfactant primarily obtained from Pseudomonas aeruginosa. By optimizing synthesis and phase transfer conditions, particles were observed to be monodispersed and stable in water under environmentally relevant pH and ionic strength values. These materials were evaluated for U(VI) removal from water at varying dissolved inorganic carbon and pH conditions. The rhamnolipid-coated iron oxide nanoparticles (IONPs) showed high sorption capacities at pH 6 and pH 8 in both carbonate-free systems and systems in equilibrium with atmospheric CO2. Equilibrium sorption behavior was interpreted using surface complexation modeling (SCM). Two models (diffuse double layer and non-electrostatic) were evaluated for their ability to account for U(VI) binding to the carboxyl groups of the rhamnolipid coating as a function of the pH, total U(VI) loading, and dissolved inorganic carbon concentration. The diffuse double layer model provided the best simulation of the adsorption data and was sensitive to U(VI) loadings as it accounted for the change in the surface charge associated with U(VI) adsorption.
中文翻译:

鼠李糖脂包裹的工程磁铁矿纳米颗粒对U(VI)吸附和分离的建模性能
基于可调节的特性,工程纳米颗粒(NPs)在水处理技术方面具有广阔的前景。出于对某些纳米颗粒的毒性和不可生物降解性的关注,我们探索了具有生物相容性涂层的工程磁铁矿(Fe 3 O 4)纳米颗粒。这些用鼠李糖脂涂层制备,鼠李糖脂是一种主要从铜绿假单胞菌获得的生物表面活性剂。通过优化合成和相转移条件,可以观察到颗粒在环境相关的pH和离子强度值下在水中单分散且稳定。这些材料的U(VI)在各种溶解的无机碳和pH条件下从水中去除。鼠李糖脂包覆的氧化铁纳米粒子(IONPs)在无碳酸盐体系和与大气CO 2平衡的体系中,在pH 6和pH 8时均显示出高吸附能力。使用表面络合模型(SCM)解释了平衡吸附行为。评估了两种模型(扩散双层和非静电模型)的能力,以解释U(VI)与鼠李糖脂涂层的羧基结合的pH值,总U(VI)含量和溶解的无机碳的函数浓度。扩散双层模型提供了最佳的吸附数据模拟,并且对U(VI)载荷,因为它解释了与U(VI)吸附相关的表面电荷的变化。
更新日期:2020-07-16
中文翻译:

鼠李糖脂包裹的工程磁铁矿纳米颗粒对U(VI)吸附和分离的建模性能
基于可调节的特性,工程纳米颗粒(NPs)在水处理技术方面具有广阔的前景。出于对某些纳米颗粒的毒性和不可生物降解性的关注,我们探索了具有生物相容性涂层的工程磁铁矿(Fe 3 O 4)纳米颗粒。这些用鼠李糖脂涂层制备,鼠李糖脂是一种主要从铜绿假单胞菌获得的生物表面活性剂。通过优化合成和相转移条件,可以观察到颗粒在环境相关的pH和离子强度值下在水中单分散且稳定。这些材料的U(VI)在各种溶解的无机碳和pH条件下从水中去除。鼠李糖脂包覆的氧化铁纳米粒子(IONPs)在无碳酸盐体系和与大气CO 2平衡的体系中,在pH 6和pH 8时均显示出高吸附能力。使用表面络合模型(SCM)解释了平衡吸附行为。评估了两种模型(扩散双层和非静电模型)的能力,以解释U(VI)与鼠李糖脂涂层的羧基结合的pH值,总U(VI)含量和溶解的无机碳的函数浓度。扩散双层模型提供了最佳的吸附数据模拟,并且对U(VI)载荷,因为它解释了与U(VI)吸附相关的表面电荷的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号