当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and solvation of confined water and water–ethanol clusters within microporous Brønsted acids and their effects on ethanol dehydration catalysis
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0sc02589e Jason S. Bates 1, 2, 3, 4 , Brandon C. Bukowski 1, 2, 3, 4 , Jeffrey Greeley 1, 2, 3, 4 , Rajamani Gounder 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-18 , DOI: 10.1039/d0sc02589e Jason S. Bates 1, 2, 3, 4 , Brandon C. Bukowski 1, 2, 3, 4 , Jeffrey Greeley 1, 2, 3, 4 , Rajamani Gounder 1, 2, 3, 4
Affiliation
|
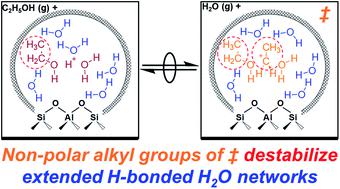
Aqueous-phase reactions within microporous Brønsted acids occur at active centers comprised of water-reactant-clustered hydronium ions, solvated within extended hydrogen-bonded water networks that tend to stabilize reactive intermediates and transition states differently. The effects of these diverse clustered and networked structures were disentangled here by measuring turnover rates of gas-phase ethanol dehydration to diethyl ether (DEE) on H-form zeolites as water pressure was increased to the point of intrapore condensation, causing protons to become solvated in larger clusters that subsequently become solvated by extended hydrogen-bonded water networks, according to in situ IR spectra. Measured first-order rate constants in ethanol quantify the stability of SN2 transition states that eliminate DEE relative to (C2H5OH)(H+)(H2O)n clusters of increasing molecularity, whose structures were respectively determined using metadynamics and ab initio molecular dynamics simulations. At low water pressures (2–10 kPa H2O), rate inhibition by water (−1 reaction order) reflects the need to displace one water by ethanol in the cluster en route to the DEE-formation transition state, which resides at the periphery of water–ethanol clusters. At higher water pressures (10–75 kPa H2O), water–ethanol clusters reach their maximum stable size ((C2H5OH)(H+)(H2O)4–5), and water begins to form extended hydrogen-bonded networks; concomitantly, rate inhibition by water (up to −3 reaction order) becomes stronger than expected from the molecularity of the reaction, reflecting the more extensive disruption of hydrogen bonds at DEE-formation transition states that contain an additional solvated non-polar ethyl group compared to the relevant reactant cluster, as described by non-ideal thermodynamic formalisms of reaction rates. Microporous voids of different hydrophilic binding site density (Beta; varying H+ and Si–OH density) and different size and shape (Beta, MFI, TON, CHA, AEI, FAU), influence the relative extents to which intermediates and transition states disrupt their confined water networks, which manifest as different kinetic orders of inhibition at high water pressures. The confinement of water within sub-nanometer spaces influences the structures and dynamics of the complexes and extended networks formed, and in turn their ability to accommodate the evolution in polarity and hydrogen-bonding capacity as reactive intermediates become transition states in Brønsted acid-catalyzed reactions.
中文翻译:

微孔布朗斯台德酸中受限水和水-乙醇簇的结构和溶剂化及其对乙醇脱水催化的影响
布伦斯台德酸微孔酸中的水相反应发生在由水反应物簇状水合氢离子组成的活性中心,在扩展的氢键水网络中被溶剂化,趋于以不同方式稳定反应性中间体和过渡态。通过测量当水压升高至孔内缩合点从而使质子溶剂化时,通过测量H型沸石上气相乙醇脱水成乙醚(DEE)的周转率,可以解开这些多样的簇状和网络结构的影响。根据原位红外光谱,在较大的团簇中,这些团簇随后被扩展的氢键水网络溶解。乙醇中测得的一级速率常数可量化S N的稳定性相对于增加分子的(C 2 H 5 OH)(H +)(H 2 O)n簇,消除了DEE的2个过渡态,它们的结构分别使用元动力学和从头算分子动力学模拟确定。在低水压(2–10 kPa H 2 O)下,水的速率抑制(-1反应阶数)反映了需要在簇中以DEE-形成过渡态(位于DEE形成态)的方式用乙醇置换一种水。水-乙醇簇的外围。在较高的水压下(10–75 kPa H 2 O),水-乙醇簇达到其最大稳定尺寸((C 2 H 5 OH)(H+)(H 2 O) 4-5),水开始形成扩展的氢键网络;同时,水的速率抑制作用(最高-3级反应)变得比反应分子预期的要强,反映出与包含另外的溶剂化非极性乙基相比,DEE形成过渡态氢键的破坏更广泛。如有关反应速率的非理想热力学形式学所述的那样,生成相应的反应簇。不同亲水结合位点密度(β; H +变化)的微孔空隙和Si-OH密度)和大小和形状(β,MFI,TON,CHA,AEI,FAU)的不同,会影响中间体和过渡态破坏其受限水网络的相对程度,这表现为不同的动力学抑制顺序高水压。水在亚纳米空间内的封闭会影响形成的络合物和扩展网络的结构和动力学,进而影响其随着极性中间体和布朗斯台德酸催化反应的过渡态而适应极性和氢键能力演变的能力。 。
更新日期:2020-07-15
中文翻译:

微孔布朗斯台德酸中受限水和水-乙醇簇的结构和溶剂化及其对乙醇脱水催化的影响
布伦斯台德酸微孔酸中的水相反应发生在由水反应物簇状水合氢离子组成的活性中心,在扩展的氢键水网络中被溶剂化,趋于以不同方式稳定反应性中间体和过渡态。通过测量当水压升高至孔内缩合点从而使质子溶剂化时,通过测量H型沸石上气相乙醇脱水成乙醚(DEE)的周转率,可以解开这些多样的簇状和网络结构的影响。根据原位红外光谱,在较大的团簇中,这些团簇随后被扩展的氢键水网络溶解。乙醇中测得的一级速率常数可量化S N的稳定性相对于增加分子的(C 2 H 5 OH)(H +)(H 2 O)n簇,消除了DEE的2个过渡态,它们的结构分别使用元动力学和从头算分子动力学模拟确定。在低水压(2–10 kPa H 2 O)下,水的速率抑制(-1反应阶数)反映了需要在簇中以DEE-形成过渡态(位于DEE形成态)的方式用乙醇置换一种水。水-乙醇簇的外围。在较高的水压下(10–75 kPa H 2 O),水-乙醇簇达到其最大稳定尺寸((C 2 H 5 OH)(H+)(H 2 O) 4-5),水开始形成扩展的氢键网络;同时,水的速率抑制作用(最高-3级反应)变得比反应分子预期的要强,反映出与包含另外的溶剂化非极性乙基相比,DEE形成过渡态氢键的破坏更广泛。如有关反应速率的非理想热力学形式学所述的那样,生成相应的反应簇。不同亲水结合位点密度(β; H +变化)的微孔空隙和Si-OH密度)和大小和形状(β,MFI,TON,CHA,AEI,FAU)的不同,会影响中间体和过渡态破坏其受限水网络的相对程度,这表现为不同的动力学抑制顺序高水压。水在亚纳米空间内的封闭会影响形成的络合物和扩展网络的结构和动力学,进而影响其随着极性中间体和布朗斯台德酸催化反应的过渡态而适应极性和氢键能力演变的能力。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号