当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study of the Properties of Acetonitrile/Water-in-Salt Hybrid Electrolytes as Electrolytes for Supercapacitors.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jpcb.0c03516 Pedro Inoue 1 , Eudes Fileti 1 , Thaciana Malaspina 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jpcb.0c03516 Pedro Inoue 1 , Eudes Fileti 1 , Thaciana Malaspina 1
Affiliation
|
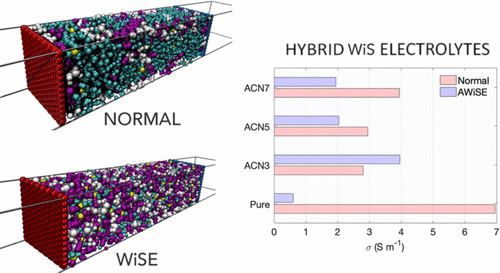
Normal and water-in-salt Li–bis(trifluoromethane) sulfonimide anion-based electrolytes were modeled using atomistic molecular dynamics simulations. Their acetonitrile (ACN) mixtures, in various concentrations, were also studied to evaluate the impact of a cosolvent on the structural, dynamical, and electrical properties of the electrolytes using liquid electrolyte and supercapacitor models. Our simulations for pure and ACN-based electrolytes revealed a drastic difference that exists between normal electrolytes and water-in-salt electrolytes and a systematic reduction of the diffusion of species by approximately a factor of 2 because of the ACN impact. Electrolytic cells for each electrolyte were built with graphene as the electrode. Our results for capacitance reveal an asymmetry between the electrode capacitances, with negative electrode capacitance systematically higher than those of the positive electrode. The total capacitance of the electrode exhibited negligible variations regardless of the concentration and composition of the electrolyte.
中文翻译:

乙腈/盐包水混合电解质作为超级电容器电解质的性能计算研究。
使用原子分子动力学模拟对普通和含盐的Li-双(三氟甲烷)磺酰亚胺阴离子型电解质进行建模。还使用液体电解质和超级电容器模型研究了其各种浓度的乙腈(ACN)混合物,以评估助溶剂对电解质的结构,动力学和电特性的影响。我们对纯和基于ACN的电解质的模拟显示,正常的电解质和盐分水电解质之间存在巨大的差异,并且由于ACN的影响,物种的扩散系统地减少了大约2倍。用石墨烯作为电极构建每种电解质的电解池。我们的电容结果揭示了电极电容之间的不对称性,负极电容系统地高于正极。不管电解质的浓度和组成如何,电极的总电容都可以忽略不计。
更新日期:2020-07-09
中文翻译:

乙腈/盐包水混合电解质作为超级电容器电解质的性能计算研究。
使用原子分子动力学模拟对普通和含盐的Li-双(三氟甲烷)磺酰亚胺阴离子型电解质进行建模。还使用液体电解质和超级电容器模型研究了其各种浓度的乙腈(ACN)混合物,以评估助溶剂对电解质的结构,动力学和电特性的影响。我们对纯和基于ACN的电解质的模拟显示,正常的电解质和盐分水电解质之间存在巨大的差异,并且由于ACN的影响,物种的扩散系统地减少了大约2倍。用石墨烯作为电极构建每种电解质的电解池。我们的电容结果揭示了电极电容之间的不对称性,负极电容系统地高于正极。不管电解质的浓度和组成如何,电极的总电容都可以忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号