当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Dynamics of a Protein Domain Relevant to the Water-Oxidizing Complex in Photosystem II as Visualized by High-Speed Atomic Force Microscopy.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c03892 Takaya Tokano 1 , Yuki Kato 1 , Shogo Sugiyama 1 , Takayuki Uchihashi 1 , Takumi Noguchi 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c03892 Takaya Tokano 1 , Yuki Kato 1 , Shogo Sugiyama 1 , Takayuki Uchihashi 1 , Takumi Noguchi 1
Affiliation
|
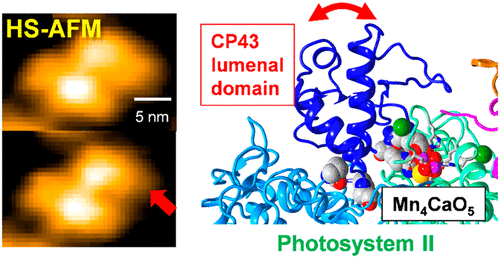
Photosystem II (PSII) is a multiprotein complex that has a function of light-driven water oxidation. The catalytic site of water oxidation is the Mn4CaO5 cluster, which is bound to the lumenal side of PSII through amino acid residues from the D1 and CP43 proteins and is further surrounded by the extrinsic proteins. In this study, we have for the first time visualized the structural dynamics of the lumenal region of a PSII core complex using high-speed atomic force microscopy (HS-AFM). The HS-AFM images of a PSII membrane fragment showed stepwise dissociation of the PsbP and PsbO extrinsic proteins. Upon subsequent destruction of the Mn4CaO5 cluster, the lumenal domain of CP43 was found to undergo a conformational fluctuation. The observed structural flexibility and conformational fluctuation of the CP43 lumenal domain are suggested to play important roles in the biogenesis of PSII and the photoassembly of the Mn4CaO5 cluster.
中文翻译:

高速原子力显微镜显示的与光系统II中水氧化复合物相关的蛋白质结构域的结构动力学。
光系统II(PSII)是一种多蛋白复合物,具有光驱动水氧化的功能。水氧化的催化位点是Mn 4 CaO 5簇,它通过D1和CP43蛋白的氨基酸残基与PSII的管腔侧结合,并进一步被外部蛋白包围。在这项研究中,我们首次使用高速原子力显微镜(HS-AFM)可视化了PSII核心复合物腔腔的结构动力学。PSII膜片段的HS-AFM图像显示了PsbP和PsbO外在蛋白的逐步解离。在随后破坏Mn 4 CaO 5时簇,发现CP43的管腔域经历构象波动。建议观察到的CP43腔结构域的结构柔性和构象波动在PSII的生物合成和Mn 4 CaO 5簇的光组装中起重要作用。
更新日期:2020-07-16
中文翻译:

高速原子力显微镜显示的与光系统II中水氧化复合物相关的蛋白质结构域的结构动力学。
光系统II(PSII)是一种多蛋白复合物,具有光驱动水氧化的功能。水氧化的催化位点是Mn 4 CaO 5簇,它通过D1和CP43蛋白的氨基酸残基与PSII的管腔侧结合,并进一步被外部蛋白包围。在这项研究中,我们首次使用高速原子力显微镜(HS-AFM)可视化了PSII核心复合物腔腔的结构动力学。PSII膜片段的HS-AFM图像显示了PsbP和PsbO外在蛋白的逐步解离。在随后破坏Mn 4 CaO 5时簇,发现CP43的管腔域经历构象波动。建议观察到的CP43腔结构域的结构柔性和构象波动在PSII的生物合成和Mn 4 CaO 5簇的光组装中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号