当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical Differences between the Binding Features of the Spike Proteins of SARS-CoV-2 and SARS-CoV.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c04317 Chen Bai 1 , Arieh Warshel 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpcb.0c04317 Chen Bai 1 , Arieh Warshel 1
Affiliation
|
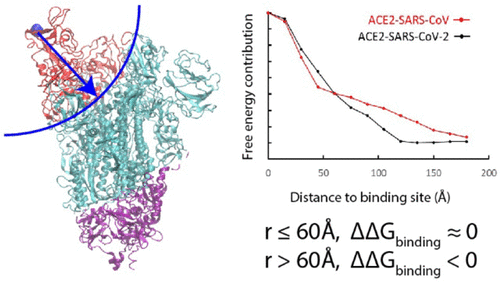
The COVID-19 caused by SARS-CoV-2 has spread globally and caused tremendous loss of lives and properties, and it is of utmost urgency to understand its propagation process and to find ways to slow down the epidemic. In this work, we used a coarse-grained model to calculate the binding free energy of SARS-CoV-2 or SARS-CoV to their human receptor ACE2. The investigation of the free energy contribution of the interacting residues indicates that the residues located outside the receptor binding domain are the source of the stronger binding of the novel virus. Thus, the current results suggest that the essential evolution of SARS-CoV-2 happens remotely from the binding domain at the spike protein trimeric body. Such evolution may facilitate the conformational change and the infection process that occurs after the virus is bound to ACE2. By studying the binding pattern between SARS-CoV antibody m396 and SARS-CoV-2, it is found that the remote energetic contribution is missing, which might explain the absence of cross-reactivity of such antibodies.
中文翻译:

SARS-CoV-2 和 SARS-CoV 刺突蛋白的结合特征之间的关键差异。
由 SARS-CoV-2 引起的 COVID-19 已在全球范围内传播,造成了巨大的生命和财产损失,了解其传播过程并找到减缓疫情的方法已刻不容缓。在这项工作中,我们使用粗粒度模型来计算 SARS-CoV-2 或 SARS-CoV 与其人类受体 ACE2 的结合自由能。对相互作用残基的自由能贡献的研究表明,位于受体结合结构域之外的残基是新型病毒更强结合的来源。因此,目前的结果表明 SARS-CoV-2 的基本进化发生在远离刺突蛋白三聚体的结合域的地方。这种进化可能会促进病毒与 ACE2 结合后发生的构象变化和感染过程。通过研究 SARS-CoV 抗体 m396 和 SARS-CoV-2 之间的结合模式,发现远程能量贡献缺失,这可能解释了此类抗体缺乏交叉反应性。
更新日期:2020-07-16
中文翻译:

SARS-CoV-2 和 SARS-CoV 刺突蛋白的结合特征之间的关键差异。
由 SARS-CoV-2 引起的 COVID-19 已在全球范围内传播,造成了巨大的生命和财产损失,了解其传播过程并找到减缓疫情的方法已刻不容缓。在这项工作中,我们使用粗粒度模型来计算 SARS-CoV-2 或 SARS-CoV 与其人类受体 ACE2 的结合自由能。对相互作用残基的自由能贡献的研究表明,位于受体结合结构域之外的残基是新型病毒更强结合的来源。因此,目前的结果表明 SARS-CoV-2 的基本进化发生在远离刺突蛋白三聚体的结合域的地方。这种进化可能会促进病毒与 ACE2 结合后发生的构象变化和感染过程。通过研究 SARS-CoV 抗体 m396 和 SARS-CoV-2 之间的结合模式,发现远程能量贡献缺失,这可能解释了此类抗体缺乏交叉反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号