当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical Properties of Re(CO)3 Complexes with and without a Local Proton Source and Implications for CO2 Reduction Catalysis
Organometallics ( IF 2.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.organomet.0c00240 Lucas A. Paul 1 , Nico C. Röttcher 1 , Jennifer Zimara 2 , Jan-Hendrik Borter 2 , Jia-Pei Du 1 , Dirk Schwarzer 2 , Ricardo A. Mata 3 , Inke Siewert 1
Organometallics ( IF 2.5 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.organomet.0c00240 Lucas A. Paul 1 , Nico C. Röttcher 1 , Jennifer Zimara 2 , Jan-Hendrik Borter 2 , Jia-Pei Du 1 , Dirk Schwarzer 2 , Ricardo A. Mata 3 , Inke Siewert 1
Affiliation
|
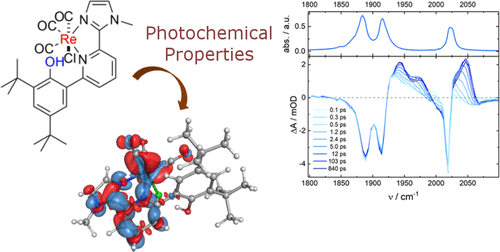
Herein, we present a detailed study on the photophysical properties and the excited state reactivity of two mononuclear Re(CO)3 complexes with imdazol-pyridine ligands equipped with and without a local proton source, [Re(CO)3LCl], where for 1: L = 2,4-ditert-butyl-6-(6-(1-methyl-1H-imidazol-2-yl)pyridin-2-yl)phenol and 2: L = 2-(3,5-ditert-butyl-2-methoxyphenyl)-6-(1-methyl-1H-imidazol-2-yl)pyridine. Time-resolved IR and UV/vis spectroscopy revealed that excitation of 1 and 2 is followed by population of the triplet excited state within <100 fs, where structural and vibrational relaxation to the T1 equilibrium structure is observed on the picosecond time scale. The T1 state can be viewed as a MLCT state as all ν(CO) features in the transient infrared (TRIR) spectra are shifted to higher wavenumbers upon excitation, which is indicative for a decreasing Re → CO π-backdonation. The T1 states have considerably long lifetimes at room temperature of 160 ns for 1 and 430 ns for 2 in dmf and they can be successfully quenched by the sacrificial electron donors triethanolamine (TEOA) and 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH). The quenching rates are 2 orders of magnitude larger for BIH than for TEOA, as the latter reaction is endergonic. However, both species are not active in the photochemical CO2-to-CO conversion. We rationalize this for 2 by the low steady-state concentration of the initial reduction product, [Re(CO)3LCl]−, which ejects chloride rather fast. Thus, the second, homogeneous electron transfer process between [Re(CO)3LCl]− and [Re(CO)3L(solvent)] forming the active species [Re(CO)3L]−, has a very low probability and decomposition pathways come to the fore. 1 decomposes under irradiation in the presence of BIH or TEOA forming the initial photoproduct 3. We tentatively assume that the ligand in 3 is deprotonated and switches from a N,N- to a N,O–-coordination mode. This indicates that in the excited state the Re–N bond is cleaved quite easily, as this decomposition pathway has not been observed under electrochemical conditions.
中文翻译:

带有和不带有局部质子源的Re(CO)3配合物的光化学性质及其对CO 2还原催化的意义
本文中,我们对带有和不带有局部质子源[Re(CO)3 LCl]的咪唑-吡啶配体的两个单核Re(CO)3配合物的光物理性质和激发态反应性进行了详细研究,其中1:L = 2,4-二叔丁基-6-(6-(1-甲基-1 H-咪唑-2-基)吡啶-2-基)苯酚和2:L = 2-(3,5 -di叔丁基-2-甲氧基苯基)-6-(1-甲基- 1 H ^ -咪唑-2-基)吡啶。时间分辨的IR和UV / Vis光谱显示激发1和2其次是<100 fs内三重激发态的填充,在皮秒级的时间范围内观察到T 1平衡结构的结构和振动弛豫。T 1状态可以看作是MLCT状态,因为瞬态红外(TRIR)光谱中的所有ν(CO)特征在激发后都移到了更高的波数,这表明Re→COπ-回递减少。T 1状态在dmf中在室温下160 ns持续1 ns和430 ns持续2 dmf的寿命相当长,并且可以通过牺牲电子供体三乙醇胺(TEOA)和1,3-二甲基-2-苯基-2来成功淬灭它们。 ,3-二氢-1 H-苯并[ d]咪唑(BIH)。BIH的淬灭速率比TEOA的淬灭速率大2个数量级,因为后者的反应是发光的。但是,这两种物质在光化学CO 2到CO的转化中都没有活性。我们通过初始还原产物[Re(CO)3 LCl] -的低稳态浓度将其合理化为2,从而相当快地排出氯化物。因此,形成活性物质[Re(CO)3 L] -的[Re(CO)3 LCl] -和[Re(CO)3 L(solvent)]之间的第二次均匀电子转移过程的可能性非常低分解途径应运而生。1个在BIH或TEOA存在下辐射下分解形成初始光产品3。我们暂时假定在配体3被去质子化,并从一个切换Ñ,Ñ -到Ñ,ö - -coordination模式。这表明在激发态,Re-N键很容易断裂,因为在电化学条件下未观察到该分解途径。
更新日期:2020-07-13
中文翻译:

带有和不带有局部质子源的Re(CO)3配合物的光化学性质及其对CO 2还原催化的意义
本文中,我们对带有和不带有局部质子源[Re(CO)3 LCl]的咪唑-吡啶配体的两个单核Re(CO)3配合物的光物理性质和激发态反应性进行了详细研究,其中1:L = 2,4-二叔丁基-6-(6-(1-甲基-1 H-咪唑-2-基)吡啶-2-基)苯酚和2:L = 2-(3,5 -di叔丁基-2-甲氧基苯基)-6-(1-甲基- 1 H ^ -咪唑-2-基)吡啶。时间分辨的IR和UV / Vis光谱显示激发1和2其次是<100 fs内三重激发态的填充,在皮秒级的时间范围内观察到T 1平衡结构的结构和振动弛豫。T 1状态可以看作是MLCT状态,因为瞬态红外(TRIR)光谱中的所有ν(CO)特征在激发后都移到了更高的波数,这表明Re→COπ-回递减少。T 1状态在dmf中在室温下160 ns持续1 ns和430 ns持续2 dmf的寿命相当长,并且可以通过牺牲电子供体三乙醇胺(TEOA)和1,3-二甲基-2-苯基-2来成功淬灭它们。 ,3-二氢-1 H-苯并[ d]咪唑(BIH)。BIH的淬灭速率比TEOA的淬灭速率大2个数量级,因为后者的反应是发光的。但是,这两种物质在光化学CO 2到CO的转化中都没有活性。我们通过初始还原产物[Re(CO)3 LCl] -的低稳态浓度将其合理化为2,从而相当快地排出氯化物。因此,形成活性物质[Re(CO)3 L] -的[Re(CO)3 LCl] -和[Re(CO)3 L(solvent)]之间的第二次均匀电子转移过程的可能性非常低分解途径应运而生。1个在BIH或TEOA存在下辐射下分解形成初始光产品3。我们暂时假定在配体3被去质子化,并从一个切换Ñ,Ñ -到Ñ,ö - -coordination模式。这表明在激发态,Re-N键很容易断裂,因为在电化学条件下未观察到该分解途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号