当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrocycle Cell Permeability Measured by Solvation Free Energies in Polar and Apolar Environments.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jcim.0c00280 Anna S Kamenik 1 , Johannes Kraml 1 , Florian Hofer 1 , Franz Waibl 1 , Patrick K Quoika 1 , Ursula Kahler 1 , Michael Schauperl 1 , Klaus R Liedl 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-18 , DOI: 10.1021/acs.jcim.0c00280 Anna S Kamenik 1 , Johannes Kraml 1 , Florian Hofer 1 , Franz Waibl 1 , Patrick K Quoika 1 , Ursula Kahler 1 , Michael Schauperl 1 , Klaus R Liedl 1
Affiliation
|
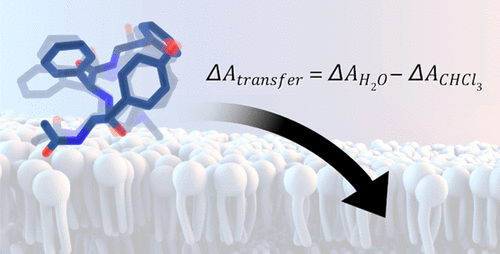
The relation of surface polarity and conformational preferences is decisive for cell permeability and thus bioavailability of macrocyclic drugs. Here, we employ grid inhomogeneous solvation theory (GIST) to calculate solvation free energies for a series of six macrocycles in water and chloroform as a measure of passive membrane permeability. We perform accelerated molecular dynamics simulations to capture a diverse structural ensemble in water and chloroform, allowing for a direct profiling of solvent-dependent conformational preferences. Subsequent GIST calculations facilitate a quantitative measure of solvent preference in the form of a transfer free energy, calculated from the ensemble-averaged solvation free energies in water and chloroform. Hence, the proposed method considers how the conformational diversity of macrocycles in polar and apolar solvents translates into transfer free energies. Following this strategy, we find a striking correlation of 0.92 between experimentally determined cell permeabilities and calculated transfer free energies. For the studied model systems, we find that the transfer free energy exceeds the purely water-based solvation free energies as a reliable estimate of cell permeability and that conformational sampling is imperative for a physically meaningful model. We thus recommend this purely physics-based approach as a computational tool to assess cell permeabilities of macrocyclic drug candidates.
中文翻译:

通过极性和非极性环境中的溶剂自由能测量的大环细胞渗透率。
表面极性和构象偏好的关系对细胞渗透性和大环药物的生物利用度具有决定性作用。在这里,我们采用网格不均匀溶剂化理论 (GIST) 来计算一系列六个大环在水中和氯仿中的溶剂化自由能,作为被动膜渗透性的量度。我们进行加速分子动力学模拟以捕获水和氯仿中的多种结构集合,从而可以直接分析溶剂依赖性构象偏好。随后的 GIST 计算有助于以转移自由能的形式定量测量溶剂偏好,该转移自由能是根据水和氯仿中的整体平均溶剂化自由能计算得出的。因此,所提出的方法考虑了极性和非极性溶剂中大环的构象多样性如何转化为转移自由能。按照这种策略,我们发现实验确定的细胞渗透率和计算的转移自由能之间存在 0.92 的显着相关性。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。92 在实验确定的细胞渗透率和计算的转移自由能之间。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。92 在实验确定的细胞渗透率和计算的转移自由能之间。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。
更新日期:2020-07-27
中文翻译:

通过极性和非极性环境中的溶剂自由能测量的大环细胞渗透率。
表面极性和构象偏好的关系对细胞渗透性和大环药物的生物利用度具有决定性作用。在这里,我们采用网格不均匀溶剂化理论 (GIST) 来计算一系列六个大环在水中和氯仿中的溶剂化自由能,作为被动膜渗透性的量度。我们进行加速分子动力学模拟以捕获水和氯仿中的多种结构集合,从而可以直接分析溶剂依赖性构象偏好。随后的 GIST 计算有助于以转移自由能的形式定量测量溶剂偏好,该转移自由能是根据水和氯仿中的整体平均溶剂化自由能计算得出的。因此,所提出的方法考虑了极性和非极性溶剂中大环的构象多样性如何转化为转移自由能。按照这种策略,我们发现实验确定的细胞渗透率和计算的转移自由能之间存在 0.92 的显着相关性。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。92 在实验确定的细胞渗透率和计算的转移自由能之间。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。92 在实验确定的细胞渗透率和计算的转移自由能之间。对于所研究的模型系统,我们发现转移自由能超过了纯水基溶剂化自由能作为细胞渗透性的可靠估计,并且构象采样对于物理上有意义的模型是必不可少的。因此,我们推荐这种纯粹基于物理的方法作为评估大环候选药物细胞渗透性的计算工具。









































 京公网安备 11010802027423号
京公网安备 11010802027423号