当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of A2V Mutation and Histidine Tautomerism on Aβ42 Monomer Structures from Atomistic Simulations.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jcim.0c00267 Hao Li 1 , Yeonsig Nam 1 , Abbas Salimi 1, 2 , Jin Yong Lee 1, 3
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jcim.0c00267 Hao Li 1 , Yeonsig Nam 1 , Abbas Salimi 1, 2 , Jin Yong Lee 1, 3
Affiliation
|
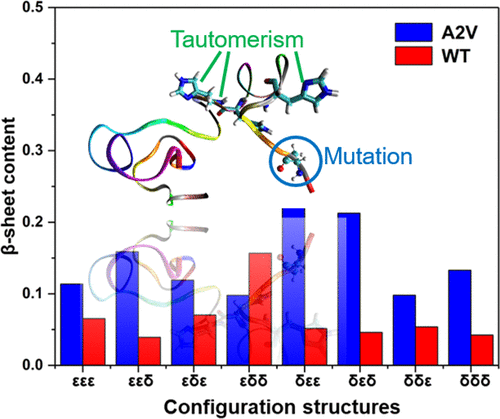
The self-assembly of amyloid-β (Aβ) peptides into senile plaques in the brain is a hallmark of Alzheimer’s disease (AD) pathology. Mutation and histidine tautomerism are considered intrinsic origins in the accumulation of Aβ. As a first step toward understanding the impact of A2V mutation and histidine tautomerism on the Aβ42 isoform, we performed replica-exchange molecular dynamics (REMD) simulations to investigate the effects of histidine tautomerism on the structural properties of A2V Aβ42 peptides. There are generally more β-sheet and less α-helix secondary structures in A2V Aβ42 monomers than in WT Aβ42, implying a higher aggregation tendency in A2V Aβ42, which is consistent with previous studies. The current research will help develop the histidine tautomerism hypothesis of misfolded protein aggregation and eventually elucidate the pathogenesis of AD.
中文翻译:

A2V突变和组氨酸互变异构对Aβ42单体结构的影响(基于原子模拟)。
淀粉样蛋白-β(Aβ)肽自组装成大脑中的老年斑是阿尔茨海默氏病(AD)病理的标志。突变和组氨酸互变异构被认为是Aβ积累的内在起源。作为了解A2V突变和组氨酸互变异构体对Aβ42亚型的影响的第一步,我们进行了副本交换分子动力学(REMD)模拟,以研究组氨酸互变异构体对A2VAβ42肽的结构特性的影响。与WTAβ42相比,A2VAβ42单体通常具有更多的β-折叠和较少的α-螺旋二级结构,这意味着A2VAβ42具有更高的聚集趋势,这与先前的研究一致。
更新日期:2020-07-27
中文翻译:

A2V突变和组氨酸互变异构对Aβ42单体结构的影响(基于原子模拟)。
淀粉样蛋白-β(Aβ)肽自组装成大脑中的老年斑是阿尔茨海默氏病(AD)病理的标志。突变和组氨酸互变异构被认为是Aβ积累的内在起源。作为了解A2V突变和组氨酸互变异构体对Aβ42亚型的影响的第一步,我们进行了副本交换分子动力学(REMD)模拟,以研究组氨酸互变异构体对A2VAβ42肽的结构特性的影响。与WTAβ42相比,A2VAβ42单体通常具有更多的β-折叠和较少的α-螺旋二级结构,这意味着A2VAβ42具有更高的聚集趋势,这与先前的研究一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号