当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Chemical Space of Synthetic Cyclic Peptides Using a Promiscuous Macrocyclase from Prenylagaramide Biosynthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acscatal.0c00623 Snigdha Sarkar 1 , Wenjia Gu 1 , Eric W Schmidt 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-17 , DOI: 10.1021/acscatal.0c00623 Snigdha Sarkar 1 , Wenjia Gu 1 , Eric W Schmidt 1
Affiliation
|
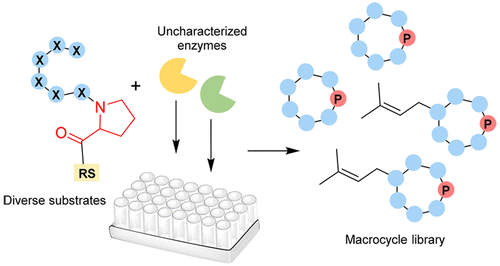
Cyclic peptides are ubiquitous drug candidates, placing macrocyclization reactions at the apex of drug development. PatG and related dual-action proteases from cyanobactin biosynthesis are responsible for cleaving off the C-terminal recognition sequence and macrocyclizing the substrate to provide cyclic peptides. This reaction has found use in the enzymatic synthesis of diverse macrocycles. However, these enzymes function best on substrates that terminate with the nonproteinogenic thiazole/thiazoline residue, complicating synthetic strategies. Here, we biochemically characterize an additional class of PatG-like macrocyclases that natively use proline, obviating the necessity of additional chemical or biochemical steps. We experimentally define the biochemical steps involved in synthesizing the widespread prenylagaramide-like natural products, including macrocyclization and prenylation. Using saturation mutagenesis, we show that macrocyclase PagG and prenyltransferase PagF are highly promiscuous, producing a library of more than 100 cyclic peptides and their prenylated derivatives in vitro. By comparing our results to known cyanobactin macrocyclases, we catalog a series of enzymes from this family that should synthesize most small macrocycles. Collectively, these data reveal that, by selecting the right cyanobactin macrocyclase, a large array of enzymatically synthesized macrocycles are accessible.
中文翻译:

使用来自异戊二烯酰胺生物合成的混杂大环化酶扩大合成环肽的化学空间
环肽是普遍存在的候选药物,将大环化反应置于药物开发的顶端。PatG 和来自氰菌素生物合成的相关双作用蛋白酶负责切割 C 末端识别序列并使底物大环化以提供环肽。该反应已用于多种大环化合物的酶促合成。然而,这些酶在以非蛋白原性噻唑/噻唑啉残基终止的底物上发挥最佳作用,使合成策略复杂化。在这里,我们对另外一类 PatG 样大环化酶进行了生化表征,它们本身使用脯氨酸,从而避免了额外的化学或生化步骤的必要性。我们通过实验定义了合成广泛的异戊二烯酰胺类天然产物所涉及的生化步骤,包括大环化和异戊二烯化。通过饱和诱变,我们发现大环化酶 PagG 和异戊烯基转移酶 PagF 是高度混杂的,在体外产生了超过 100 种环肽及其异戊二烯化衍生物的文库。通过将我们的结果与已知的氰菌素大环酶进行比较,我们对这个家族中应该合成大多数小大环化合物的一系列酶进行了分类。总的来说,这些数据表明,通过选择正确的氰菌素大环化酶,可以获得大量酶促合成的大环化合物。
更新日期:2020-07-02
中文翻译:

使用来自异戊二烯酰胺生物合成的混杂大环化酶扩大合成环肽的化学空间
环肽是普遍存在的候选药物,将大环化反应置于药物开发的顶端。PatG 和来自氰菌素生物合成的相关双作用蛋白酶负责切割 C 末端识别序列并使底物大环化以提供环肽。该反应已用于多种大环化合物的酶促合成。然而,这些酶在以非蛋白原性噻唑/噻唑啉残基终止的底物上发挥最佳作用,使合成策略复杂化。在这里,我们对另外一类 PatG 样大环化酶进行了生化表征,它们本身使用脯氨酸,从而避免了额外的化学或生化步骤的必要性。我们通过实验定义了合成广泛的异戊二烯酰胺类天然产物所涉及的生化步骤,包括大环化和异戊二烯化。通过饱和诱变,我们发现大环化酶 PagG 和异戊烯基转移酶 PagF 是高度混杂的,在体外产生了超过 100 种环肽及其异戊二烯化衍生物的文库。通过将我们的结果与已知的氰菌素大环酶进行比较,我们对这个家族中应该合成大多数小大环化合物的一系列酶进行了分类。总的来说,这些数据表明,通过选择正确的氰菌素大环化酶,可以获得大量酶促合成的大环化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号