当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rich Bismuth‐Oxygen Bonds in Bismuth Derivatives from Bi2S3 Pre‐Catalysts Promote the Electrochemical Reduction of CO2
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-17 , DOI: 10.1002/celc.202000656 Yating Wang 1 , Ling Cheng 1 , Jinze Liu 2 , Chuqian Xiao 1 , Bei Zhang 1 , Qionghao Xiong 1 , Tao Zhang 1 , Zilong Jiang 1 , Hao Jiang 1, 2 , Yihua Zhu 1 , Yuhang Li 1 , Chunzhong Li 1, 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-06-17 , DOI: 10.1002/celc.202000656 Yating Wang 1 , Ling Cheng 1 , Jinze Liu 2 , Chuqian Xiao 1 , Bei Zhang 1 , Qionghao Xiong 1 , Tao Zhang 1 , Zilong Jiang 1 , Hao Jiang 1, 2 , Yihua Zhu 1 , Yuhang Li 1 , Chunzhong Li 1, 2
Affiliation
|
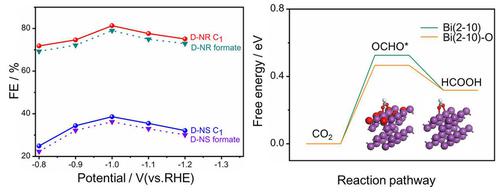
The greenhouse effect and climate change caused by carbon dioxide (CO2) have become global challenges. Electrochemical reduction is one of the most promising paths to convert CO2 into a feedstock such as formate for the synthesis of value‐added chemicals and fuels. We first performed theoretical calculations to predict the activity of bismuth oxide (Bi2O3) for CO2 electroreduction. The analysis of the reaction energetics indicates that the increased number of Bi−O bonds lowers the formation energy of the key *OCHO intermediate, and thus improves the production of formate. The Bi/Bi2O3 electrocatalyst rich in Bi−O bonds was experimentally prepared from a nanorod‐like Bi2S3 pre‐catalyst, which shows electrocatalytic activity to generate formate with a Faradaic efficiency of over 80 % at −1.0 V vs. the reversible hydrogen electrode (RHE) and durability for more than 20 h. This research opens up avenues for the rational design of selective Bi‐based CO2‐to‐formate electrocatalysts.
中文翻译:

Bi2S3预催化剂中铋衍生物中丰富的铋氧键促进电化学还原CO2
由二氧化碳(CO 2)引起的温室效应和气候变化已成为全球挑战。电化学还原是将CO 2转化为甲酸酯等原料以合成增值化学品和燃料的最有希望的途径之一。我们首先进行了理论计算,以预测氧化铋(Bi 2 O 3)对CO 2电还原的活性。反应能量学分析表明,增加的Bi-O键数目降低了关键* OCHO中间体的形成能,从而提高了甲酸酯的产生。Bi / Bi 2 O 3富含Bi-O键的电催化剂是由类似纳米棒的Bi 2 S 3预催化剂制备的,与可逆氢电极(RHE)相比,该催化剂在-1.0 V时具有电催化活性,可生成甲酸盐,法拉第效率超过80%。 ),使用寿命超过20小时。这项研究为选择性设计基于Bi的CO 2形成甲酸的电催化剂的合理设计开辟了途径。
更新日期:2020-07-06
中文翻译:

Bi2S3预催化剂中铋衍生物中丰富的铋氧键促进电化学还原CO2
由二氧化碳(CO 2)引起的温室效应和气候变化已成为全球挑战。电化学还原是将CO 2转化为甲酸酯等原料以合成增值化学品和燃料的最有希望的途径之一。我们首先进行了理论计算,以预测氧化铋(Bi 2 O 3)对CO 2电还原的活性。反应能量学分析表明,增加的Bi-O键数目降低了关键* OCHO中间体的形成能,从而提高了甲酸酯的产生。Bi / Bi 2 O 3富含Bi-O键的电催化剂是由类似纳米棒的Bi 2 S 3预催化剂制备的,与可逆氢电极(RHE)相比,该催化剂在-1.0 V时具有电催化活性,可生成甲酸盐,法拉第效率超过80%。 ),使用寿命超过20小时。这项研究为选择性设计基于Bi的CO 2形成甲酸的电催化剂的合理设计开辟了途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号