当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Interaction between HIV-1 Protease and the Homodimeric p66/p66' Reverse Transcriptase Precursor by Double Electron-Electron Resonance EPR Spectroscopy.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-06-18 , DOI: 10.1002/cbic.202000263 Thomas Schmidt 1 , John M Louis 1 , G Marius Clore 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-06-18 , DOI: 10.1002/cbic.202000263 Thomas Schmidt 1 , John M Louis 1 , G Marius Clore 1
Affiliation
|
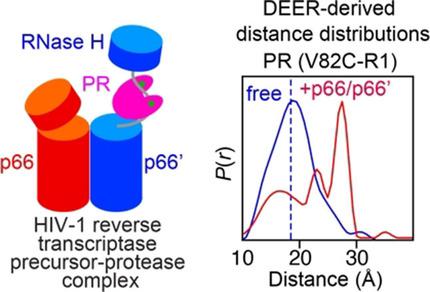
Opening up p66: Q‐band pulsed double electron‐electron resonance EPR spectroscopy shows that binding of HIV‐1 protease to the asymmetric p66/p66′ homodimer precursor of HIV‐1 reverse transcriptase favours an open conformation of the p66′ subunit allowing the linker connecting the RNaseH and connection domains to bind to the active site of protease in an extended conformation.

中文翻译:

通过双电子共振 EPR 光谱探讨 HIV-1 蛋白酶与同源二聚体 p66/p66' 逆转录酶前体之间的相互作用。
打开p66 :Q带脉冲双电子共振EPR光谱表明,HIV-1蛋白酶与HIV-1逆转录酶的不对称p66/p66'同二聚体前体的结合有利于p66'亚基的开放构象,从而允许连接子连接 RNaseH 和连接域,以扩展构象与蛋白酶的活性位点结合。

更新日期:2020-06-18

中文翻译:

通过双电子共振 EPR 光谱探讨 HIV-1 蛋白酶与同源二聚体 p66/p66' 逆转录酶前体之间的相互作用。
打开p66 :Q带脉冲双电子共振EPR光谱表明,HIV-1蛋白酶与HIV-1逆转录酶的不对称p66/p66'同二聚体前体的结合有利于p66'亚基的开放构象,从而允许连接子连接 RNaseH 和连接域,以扩展构象与蛋白酶的活性位点结合。










































 京公网安备 11010802027423号
京公网安备 11010802027423号