当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-dimensional imaging and quantification of real-time cytosolic calcium oscillations in microglial cells cultured on electrospun matrices using laser scanning confocal microscopy.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-18 , DOI: 10.1002/bit.27465 Kojja Venkateswarlu 1 , Gare Suman 1 , Vaibhav Dhyani 1 , Sarpras Swain 1 , Lopamudra Giri 1 , Satyavrata Samavedi 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-06-18 , DOI: 10.1002/bit.27465 Kojja Venkateswarlu 1 , Gare Suman 1 , Vaibhav Dhyani 1 , Sarpras Swain 1 , Lopamudra Giri 1 , Satyavrata Samavedi 1
Affiliation
|
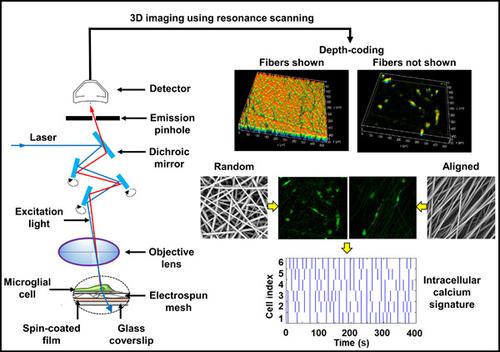
The development of a minimally invasive, robust, and inexpensive technique that permits real‐time monitoring of cell responses on biomaterial scaffolds can improve the eventual outcomes of scaffold‐based tissue engineering strategies. Towards establishing correlations between in situ biological activity and cell fate, we have developed a comprehensive workflow for real‐time volumetric imaging of spatiotemporally varying cytosolic calcium oscillations in pure microglial cells cultured on electrospun meshes. Live HMC3 cells on randomly oriented electrospun fibers were stained with a fluorescent dye and imaged using a laser scanning confocal microscope. Resonance scanning provided high‐resolution in obtaining the time‐course of intracellular calcium levels without compromising spatial and temporal resolution. Three‐dimensional reconstruction and depth‐coding enabled the visualization of cell location and intracellular calcium levels as a function of sample thickness. Importantly, changes in cell morphology and in situ calcium spiking were quantified in response to a soluble biochemical cue and varying matrix architectures (i.e., randomly oriented and aligned fibers). Importantly, raster plots generated from spiking data revealed calcium signatures specific to culture conditions. In the future, our approach can be used to elucidate correlations between calcium signatures and cell phenotype/activation, and facilitate the rational design of scaffolds for biomedical applications.
中文翻译:

使用激光扫描共聚焦显微镜在电纺基质上培养的小胶质细胞中实时细胞溶质钙振荡的三维成像和量化。
开发一种微创、稳健且廉价的技术,允许实时监测生物材料支架上的细胞反应,可以改善基于支架的组织工程策略的最终结果。为了建立原位生物活性和细胞命运之间的相关性,我们开发了一个全面的工作流程,用于对电纺网培养的纯小胶质细胞中时空变化的细胞溶质钙振荡进行实时体积成像。随机定向的电纺纤维上的活 HMC3 细胞用荧光染料染色,并使用激光扫描共聚焦显微镜成像。共振扫描在获得细胞内钙水平的时间过程方面提供了高分辨率,而不会影响空间和时间分辨率。三维重建和深度编码使细胞位置和细胞内钙水平可视化为样品厚度的函数。重要的是,响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。
更新日期:2020-06-18
中文翻译:

使用激光扫描共聚焦显微镜在电纺基质上培养的小胶质细胞中实时细胞溶质钙振荡的三维成像和量化。
开发一种微创、稳健且廉价的技术,允许实时监测生物材料支架上的细胞反应,可以改善基于支架的组织工程策略的最终结果。为了建立原位生物活性和细胞命运之间的相关性,我们开发了一个全面的工作流程,用于对电纺网培养的纯小胶质细胞中时空变化的细胞溶质钙振荡进行实时体积成像。随机定向的电纺纤维上的活 HMC3 细胞用荧光染料染色,并使用激光扫描共聚焦显微镜成像。共振扫描在获得细胞内钙水平的时间过程方面提供了高分辨率,而不会影响空间和时间分辨率。三维重建和深度编码使细胞位置和细胞内钙水平可视化为样品厚度的函数。重要的是,响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。响应可溶性生化线索和不同的基质结构(即随机定向和排列的纤维),量化细胞形态和原位钙尖峰的变化。重要的是,从加标数据生成的栅格图揭示了特定于培养条件的钙特征。未来,我们的方法可用于阐明钙特征与细胞表型/激活之间的相关性,并促进生物医学应用支架的合理设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号