当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemically Modifying the Electronic Structure of IrO2 Nanoparticles for Overall Electrochemical Water Splitting with Extensive Adaptability
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/aenm.202001600 Lu Li 1 , Bin Wang 1 , Gengwei Zhang 1 , Guang Yang 2 , Tao Yang 1 , Sen Yang 1 , Shengchun Yang 1, 3
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-06-18 , DOI: 10.1002/aenm.202001600 Lu Li 1 , Bin Wang 1 , Gengwei Zhang 1 , Guang Yang 2 , Tao Yang 1 , Sen Yang 1 , Shengchun Yang 1, 3
Affiliation
|
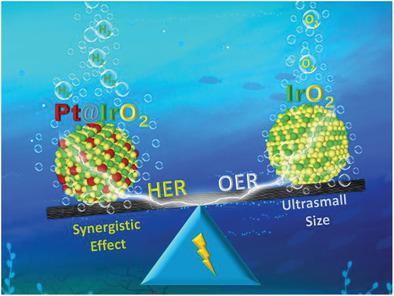
Designing the electrocatalysts that are stable and active for extensively adaptable water splitting is highly desirable for developing hydrogen based energy. IrO2 is a promising and widely used catalyst for the oxygen evolution reaction in commercial applications, but is rarely used for the hydrogen evolution reaction (HER), due to the high Gibbs free energy for hydrogen adsorption (ΔG H*). Herein, an approach to modify the electronic structure of IrO2 via cyclic voltammetry is proposed. In this process, Ir(+4) is partially reduced and trace Pt is simultaneously deposited on IrO2, which greatly lowers the ΔG H* and thus accelerates the reaction kinetics. The as‐prepared Pt–IrO2/CC with low noble metal loading (36.6 µg cm−2(Ir+Pt)) exhibits excellent HER activity with overpotentials of 5, 22, and 26 mV at 10 mA cm−2 in 0.5 m H2SO4, 1 m KOH, and 1 m phosphate buffer solution, respectively, making it possible to organize an all‐IrO2 based water electrolyzer. The Pt–IrO2/CC||IrO2/CC couple exhibits a promising activity and stability in pH‐universal conditions as well as natural seawater for H2 production. Density function theory calculations reveal that the optimized electronic structure of IrO2 balances the ΔG H*, resulting in a much enhanced HER performance.
中文翻译:

电化学修饰IrO2纳米粒子的电子结构,以实现广泛的整体水分解能力
为了开发基于氢的能量,非常需要设计对于广泛适用的水分解而言稳定且有活性的电催化剂。IrO 2是用于工业应用中的氧释放反应的有希望且广泛使用的催化剂,但是由于用于氢吸附的高吉布斯自由能(ΔG H *),因此很少用于氢释放反应(HER )。在此,提出了通过循环伏安法改变IrO 2的电子结构的方法。在此过程中,Ir(+4)被部分还原,同时痕量Pt同时沉积在IrO 2上,这大大降低了ΔG H *,从而加快了反应动力学。准备好的Pt–IrO2具有低的贵金属负载/ CC(36.6微克厘米-2 (IR + Pt)的)表现出优异的HER为5,22和26毫伏在10mA厘米的超电势活性-2 0.5米ħ 2 SO 4,1个米分别使用KOH和1 m磷酸盐缓冲溶液,可以组织基于全IrO 2的水电解槽。Pt–IrO 2 / CC || IrO 2 / CC对在pH通用条件下以及天然海水生产H 2中均显示出良好的活性和稳定性。密度泛函理论计算表明,优化的IrO电子结构图2平衡了ΔG H *,从而大大提高了HER性能。
更新日期:2020-08-11
中文翻译:

电化学修饰IrO2纳米粒子的电子结构,以实现广泛的整体水分解能力
为了开发基于氢的能量,非常需要设计对于广泛适用的水分解而言稳定且有活性的电催化剂。IrO 2是用于工业应用中的氧释放反应的有希望且广泛使用的催化剂,但是由于用于氢吸附的高吉布斯自由能(ΔG H *),因此很少用于氢释放反应(HER )。在此,提出了通过循环伏安法改变IrO 2的电子结构的方法。在此过程中,Ir(+4)被部分还原,同时痕量Pt同时沉积在IrO 2上,这大大降低了ΔG H *,从而加快了反应动力学。准备好的Pt–IrO2具有低的贵金属负载/ CC(36.6微克厘米-2 (IR + Pt)的)表现出优异的HER为5,22和26毫伏在10mA厘米的超电势活性-2 0.5米ħ 2 SO 4,1个米分别使用KOH和1 m磷酸盐缓冲溶液,可以组织基于全IrO 2的水电解槽。Pt–IrO 2 / CC || IrO 2 / CC对在pH通用条件下以及天然海水生产H 2中均显示出良好的活性和稳定性。密度泛函理论计算表明,优化的IrO电子结构图2平衡了ΔG H *,从而大大提高了HER性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号