Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-06-18 , DOI: 10.1016/j.jechem.2020.06.012 Qiyou Wang , Yang Gao , Zhongyun Ma , Yan Zhang , Wenpeng Ni , Hussein A. Younus , Ce Zhang , Zhengjian Chen , Shiguo Zhang
|
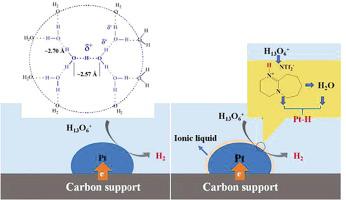
Platinum is generally known as the most effective electrocatalyst for hydrogen evolution reaction because it can greatly lower the overpotential and accelerate the reaction kinetics, while its commercial potential always suffers from scarcity, high cost, low utilization, and poor durability particularly in acidic electrolytes. We herein demonstrate a facile method to improve the hydrogen evolution performance of Pt-based electrocatalysts by simply decorating the-state-of-the-art and commercially available Pt/C with hydrophobic protic ([DBU][NTf2]) or aprotic ([BMIm][NTf2]) ionic liquid. The current densities of [BMIm]@Pt/C and [DBU-H]@Pt/C with 10% ionic liquid at an overpotential of 40 mV are 2.81 and 4.15 times, respectively, higher than that of the pristine Pt/C. More importantly, ionic liquid-decoration significantly improves the long-term stability of Pt nanoparticles. After 8 h of chronoamperometric measurements, [DBU-H]@Pt/C and [BMIm]@Pt/C can still retain 83.7% and 78.3% of their original activity, respectively, which is much higher than that of the pristine Pt/C (24.4%). The improved performance of Pt/C decorated with ionic liquid is considered to arise from the improved proton conductivity (particularly for protic ionic liquid) and hydrophobic microenvironment created by the supported ionic liquid phase. The presence of ionic liquid layer not only de-coordinates H+ from hydronium ions nearby the Pt nanoparticles, but it also protects Pt nanoparticles from dissolution in the acidic media.
中文翻译:

负载型离子液体增强的高活性和持久性电催化剂,可在酸性电解质中发生氢释放反应
铂通常被认为是最有效的氢气释放反应的电催化剂,因为它可以大大降低过电位并加速反应动力学,而其商业潜力却始终缺乏,尤其是在酸性电解质中稀缺,成本高,利用率低和耐久性差。我们在本文中演示了一种简便的方法,可以通过简单地用疏水性质子([DBU] [NTf 2 ])或非质子性([DBU] [NTf 2 ])装饰最新技术和市售Pt / C来提高Pt基电催化剂的氢释放性能。[BMIm] [NTf 2])离子液体。在40 mV的超电势下,含10%离子液体的[BMIm] @ Pt / C和[DBU-H] @ Pt / C的电流密度分别是原始Pt / C的2.81和4.15倍。更重要的是,离子液体修饰显着改善了Pt纳米粒子的长期稳定性。计时电流法测量8小时后,[DBU-H] @ Pt / C和[BMIm] @ Pt / C仍可分别保留其原始活性的83.7%和78.3%,远高于原始Pt / C(24.4%)。认为用离子液体修饰的Pt / C的性能提高是由于质子电导率的提高(尤其是质子离子液体)和负载型离子液相产生的疏水微环境引起的。离子液体层的存在不仅会使H +脱离坐标 不会从Pt纳米颗粒附近的水合氢离子中分离出来,但是它也可以保护Pt纳米颗粒不溶于酸性介质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号