Cell ( IF 45.5 ) Pub Date : 2020-06-18 , DOI: 10.1016/j.cell.2020.05.035 Jessica Sook Yuin Ho 1 , Matthew Angel 2 , Yixuan Ma 1 , Elizabeth Sloan 3 , Guojun Wang 4 , Carles Martinez-Romero 5 , Marta Alenquer 6 , Vladimir Roudko 7 , Liliane Chung 8 , Simin Zheng 1 , Max Chang 9 , Yesai Fstkchyan 1 , Sara Clohisey 8 , Adam M Dinan 10 , James Gibbs 2 , Robert Gifford 3 , Rong Shen 11 , Quan Gu 3 , Nerea Irigoyen 10 , Laura Campisi 1 , Cheng Huang 12 , Nan Zhao 1 , Joshua D Jones 10 , Ingeborg van Knippenberg 3 , Zeyu Zhu 1 , Natasha Moshkina 1 , Léa Meyer 3 , Justine Noel 1 , Zuleyma Peralta 13 , Veronica Rezelj 3 , Robyn Kaake 14 , Brad Rosenberg 1 , Bo Wang 8 , Jiajie Wei 2 , Slobodan Paessler 12 , Helen M Wise 8 , Jeffrey Johnson 15 , Alessandro Vannini 16 , Maria João Amorim 6 , J Kenneth Baillie 8 , Emily R Miraldi 17 , Christopher Benner 9 , Ian Brierley 10 , Paul Digard 8 , Marta Łuksza 13 , Andrew E Firth 10 , Nevan Krogan 14 , Benjamin D Greenbaum 7 , Megan K MacLeod 18 , Harm van Bakel 13 , Adolfo Garcìa-Sastre 5 , Jonathan W Yewdell 2 , Edward Hutchinson 3 , Ivan Marazzi 4
|
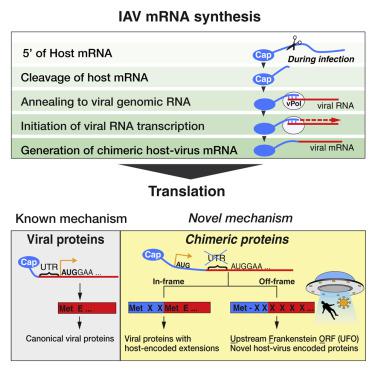
RNA viruses are a major human health threat. The life cycles of many highly pathogenic RNA viruses like influenza A virus (IAV) and Lassa virus depends on host mRNA, because viral polymerases cleave 5′-m7G-capped host transcripts to prime viral mRNA synthesis (“cap-snatching”). We hypothesized that start codons within cap-snatched host transcripts could generate chimeric human-viral mRNAs with coding potential. We report the existence of this mechanism of gene origination, which we named “start-snatching.” Depending on the reading frame, start-snatching allows the translation of host and viral “untranslated regions” (UTRs) to create N-terminally extended viral proteins or entirely novel polypeptides by genetic overprinting. We show that both types of chimeric proteins are made in IAV-infected cells, generate T cell responses, and contribute to virulence. Our results indicate that during infection with IAV, and likely a multitude of other human, animal and plant viruses, a host-dependent mechanism allows the genesis of hybrid genes.
中文翻译:

杂交基因起源在感染过程中产生人类病毒嵌合蛋白。
RNA病毒是人类健康的主要威胁。许多高致病性 RNA 病毒(例如甲型流感病毒 (IAV) 和拉沙病毒)的生命周期取决于宿主 mRNA,因为病毒聚合酶会切割 5'-m7G 加帽的宿主转录本以引发病毒 mRNA 合成(“抢帽”)。我们假设,抢夺宿主转录本中的起始密码子可以产生具有编码潜力的嵌合人病毒 mRNA。我们报告了这种基因起源机制的存在,我们将其命名为“抢夺起始”。根据阅读框,起始抢夺允许宿主和病毒“非翻译区”(UTR)的翻译,通过基因套印产生 N 末端延伸的病毒蛋白或全新的多肽。我们证明这两种类型的嵌合蛋白都是在 IAV 感染的细胞中产生的,产生 T 细胞反应,并有助于毒力。我们的结果表明,在 IAV 以及可能的许多其他人类、动物和植物病毒感染期间,宿主依赖性机制允许杂合基因的发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号