当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Innovative Impurity profiling of Avanafil using LC and LC-MS/MS with in-silico toxicity prediction
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.arabjc.2020.06.007 Patel Mital , Kothari Charmy , Vyas Vivek
Arabian Journal of Chemistry ( IF 6 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.arabjc.2020.06.007 Patel Mital , Kothari Charmy , Vyas Vivek
|
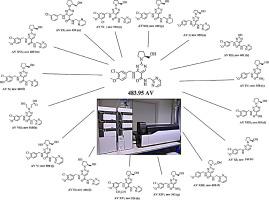
Abstract Degradation of the drug can lead to the formation of toxic substance hence drug quality and stability is a major concern by pharma regulators. A Selected phosphodiesterase type 5 inhibitor drug Avanafil (AV) structure has amide, arylchloro and hydroxide as functional groups which can easily eliminated during hydrolysis. Henceforth, thoroughly chemical stability of AV was carried out according to ICH guideline Q1A (R2). The drug and marketed tablet formulation undergoes degradation in hydrolytic (acid, base, neutral), thermal, photolytic, oxidative conditions and forms a total new sixteen degradation products (D.P.s) which were identified by LC, characterized by LC-MS/MS and probable degradation mechanism for each stress conditions are proposed. All sixteen D.P.s were identified by optimized LC conditions; C18 column using 10 mM ammonium acetate: ACN (60:40, v/v), pH 4.5 as mobile phase at 0.9 mL min-1 of flow rate, 239 nm wavelength at 20 °C column temperature and the method being LC-MS compatible characterized by LC-MS/MS confirmed by relative retention time (RRT). The structure of D.P.s was confirmed from the fragmentation pattern obtained by LC-MS/MS and further probable degradation mechanism for each stress condition is proposed. The drug and its marketed tablet formulation showed similar degradation peaks which were confirmed using RRT, photodiode array (PDA) and LC-MS. Drug degradation happens due to nucleophilic substitution reaction, amide hydrolysis, electron withdrawing properties of amide, dechlorination and bond cleavage due to energy. The amide group in AV structure can undergo hydrolysis, while due to aryl chloride and hydroxide group in structure it undergoes photodecomposition. A comprehensive stress study reveals that AV is more prone to degrade in light, temperature and moisture; hence AV requires proper storage condition temperature below 25 °C with protection to light and moisture. In silico toxicity prediction of physicochemical properties revealed that all the physicochemical parameters of impurities were within the acceptable limit which indicates that no impurity is at any risk of toxicity. In detail, the LC-MS/MS compatible AV degradation study is fully validated as per ICH Q2 (R1) guideline.
中文翻译:

使用 LC 和 LC-MS/MS 对 Avanafil 进行创新的杂质分析,并进行计算机毒性预测
摘要 药物的降解会导致有毒物质的形成,因此药物质量和稳定性是制药监管机构的主要关注点。选定的 5 型磷酸二酯酶抑制剂药物 Avanafil (AV) 结构具有酰胺、芳基氯和氢氧化物作为官能团,在水解过程中很容易消除。此后,根据 ICH 指南 Q1A (R2) 对 AV 进行彻底的化学稳定性。药物和上市片剂在水解(酸、碱、中性)、热、光解、氧化条件下发生降解,形成总共 16 种新的降解产物 (DP),经 LC 鉴定,LC-MS/MS 表征和可能提出了每种应力条件的退化机制。所有 16 个 DP 均通过优化的 LC 条件进行鉴定;C18 柱使用 10 mM 醋酸铵:乙腈(60:40,v/v),pH 4.5 作为流动相,流速为 0.9 mL min-1,20 °C 柱温下波长为 239 nm,方法为 LC-MS通过相对保留时间 (RRT) 确认的 LC-MS/MS 表征。DPs 的结构通过 LC-MS/MS 获得的碎片模式得到证实,并提出了每种应力条件下进一步可能的降解机制。该药物及其上市的片剂配方显示出类似的降解峰,使用 RRT、光电二极管阵列 (PDA) 和 LC-MS 证实了这一点。药物降解是由于亲核取代反应、酰胺水解、酰胺的吸电子特性、脱氯和能量引起的键断裂而发生的。AV结构中的酰胺基团可以发生水解,而由于结构上的芳基氯和羟基,它会发生光分解。一项全面的压力研究表明,AV 在光照、温度和湿度条件下更容易降解;因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。
更新日期:2020-08-01
中文翻译:

使用 LC 和 LC-MS/MS 对 Avanafil 进行创新的杂质分析,并进行计算机毒性预测
摘要 药物的降解会导致有毒物质的形成,因此药物质量和稳定性是制药监管机构的主要关注点。选定的 5 型磷酸二酯酶抑制剂药物 Avanafil (AV) 结构具有酰胺、芳基氯和氢氧化物作为官能团,在水解过程中很容易消除。此后,根据 ICH 指南 Q1A (R2) 对 AV 进行彻底的化学稳定性。药物和上市片剂在水解(酸、碱、中性)、热、光解、氧化条件下发生降解,形成总共 16 种新的降解产物 (DP),经 LC 鉴定,LC-MS/MS 表征和可能提出了每种应力条件的退化机制。所有 16 个 DP 均通过优化的 LC 条件进行鉴定;C18 柱使用 10 mM 醋酸铵:乙腈(60:40,v/v),pH 4.5 作为流动相,流速为 0.9 mL min-1,20 °C 柱温下波长为 239 nm,方法为 LC-MS通过相对保留时间 (RRT) 确认的 LC-MS/MS 表征。DPs 的结构通过 LC-MS/MS 获得的碎片模式得到证实,并提出了每种应力条件下进一步可能的降解机制。该药物及其上市的片剂配方显示出类似的降解峰,使用 RRT、光电二极管阵列 (PDA) 和 LC-MS 证实了这一点。药物降解是由于亲核取代反应、酰胺水解、酰胺的吸电子特性、脱氯和能量引起的键断裂而发生的。AV结构中的酰胺基团可以发生水解,而由于结构上的芳基氯和羟基,它会发生光分解。一项全面的压力研究表明,AV 在光照、温度和湿度条件下更容易降解;因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。因此,AV 需要适当的储存条件温度低于 25 °C,并保护光线和水分。理化性质的计算机毒性预测表明,杂质的所有理化参数都在可接受的范围内,这表明没有杂质有任何毒性风险。具体而言,LC-MS/MS 兼容的 AV 降解研究已根据 ICH Q2 (R1) 指南进行了充分验证。



























 京公网安备 11010802027423号
京公网安备 11010802027423号