当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocathode-assisted redox flow desalination
Green Chemistry ( IF 9.3 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0gc01191f Mengjun Liang 1, 2, 3, 4, 5 , Kuang Feng 5, 6, 7, 8 , Ramalingam Karthick 1, 2, 3, 4, 5 , Liguo Zhang 1, 2, 3, 4, 5 , Yumeng Shi 8, 9, 10, 11, 12 , Kwan San Hui 13, 14, 15, 16, 17 , Kwun Nam Hui 18, 19, 20, 21, 22 , Feng Jiang 5, 6, 7, 8 , Fuming Chen 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0gc01191f Mengjun Liang 1, 2, 3, 4, 5 , Kuang Feng 5, 6, 7, 8 , Ramalingam Karthick 1, 2, 3, 4, 5 , Liguo Zhang 1, 2, 3, 4, 5 , Yumeng Shi 8, 9, 10, 11, 12 , Kwan San Hui 13, 14, 15, 16, 17 , Kwun Nam Hui 18, 19, 20, 21, 22 , Feng Jiang 5, 6, 7, 8 , Fuming Chen 1, 2, 3, 4, 5
Affiliation
|
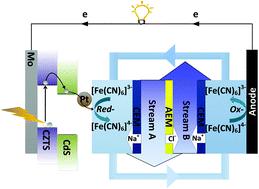
Desalination techniques, such as reverse osmosis, distillation, capacitive deionization, and battery desalination, require lots of electrical or thermal energy consumption. Herein, we propose a consumption-free electrochemical desalination method based on a light-driven photocathode with a Pt/CdS/Cu2ZnSnS4(CZTS)/Mo architecture. Modification of a CdS layer on CZTS can improve the desalination performance due to the formation of inner p–n junction between CdS and CZTS which enhances the separation of the photoexcited carriers without recombination. This photocathode-assisted electrodialysis desalination plays the dual functions of both energy conversion and ion removal with the blocking of ion exchange membranes. The [Fe(CN)6]3−/4− redox couples are recirculated between the anode and photo-cathode as the electrolyte while the salt streams are fed into the middle compartment. Under light illumination, this architecture produces photo-generated electrons to the redox couples with the conversion of [Fe(CN)6]3− to [Fe(CN)6]4− at the positive chamber, causing cation capture in the presence of an ion-exchange membrane. At the same time, [Fe(CN)6]4− is oxidized at the negative reservoir. The light-driven electrochemical reaction of electrolyte redox couples can result in a continuous desalination process. This work will be significant for consumption-free photoelectrochemical desalination research.
中文翻译:

光阴极辅助氧化还原流脱盐
诸如反渗透,蒸馏,电容去离子和电池脱盐之类的脱盐技术需要大量的电能或热能消耗。在这里,我们提出了一种基于光驱动的具有Pt / CdS / Cu 2 ZnSnS 4(CZTS)/ Mo结构的光电阴极的无消耗电化学脱盐方法。在CZTS上修改CdS层可以改善脱盐性能,这是因为在CdS和CZTS之间形成了内部p–n结,从而增强了光激发载流子的分离而无需重组。这种光阴极辅助的电渗析脱盐具有能量转换和离子交换膜封闭的双重功能。[Fe(CN)6 ] 3-/-4-当盐流被送入中间室时,氧化还原对作为电解质在阳极和光阴极之间循环。在光线照射下,这种结构会在正腔处将光生电子生成氧化还原对,并由[Fe(CN)6 ] 3−转换为[Fe(CN)6 ] 4−,从而在存在以下离子的情况下引起阳离子捕获离子交换膜。同时,[Fe(CN)6 ] 4-在负储层被氧化。电解质氧化还原对的光驱动电化学反应可导致连续的脱盐过程。这项工作对无消耗光电化学脱盐研究具有重要意义。
更新日期:2020-07-06
中文翻译:

光阴极辅助氧化还原流脱盐
诸如反渗透,蒸馏,电容去离子和电池脱盐之类的脱盐技术需要大量的电能或热能消耗。在这里,我们提出了一种基于光驱动的具有Pt / CdS / Cu 2 ZnSnS 4(CZTS)/ Mo结构的光电阴极的无消耗电化学脱盐方法。在CZTS上修改CdS层可以改善脱盐性能,这是因为在CdS和CZTS之间形成了内部p–n结,从而增强了光激发载流子的分离而无需重组。这种光阴极辅助的电渗析脱盐具有能量转换和离子交换膜封闭的双重功能。[Fe(CN)6 ] 3-/-4-当盐流被送入中间室时,氧化还原对作为电解质在阳极和光阴极之间循环。在光线照射下,这种结构会在正腔处将光生电子生成氧化还原对,并由[Fe(CN)6 ] 3−转换为[Fe(CN)6 ] 4−,从而在存在以下离子的情况下引起阳离子捕获离子交换膜。同时,[Fe(CN)6 ] 4-在负储层被氧化。电解质氧化还原对的光驱动电化学反应可导致连续的脱盐过程。这项工作对无消耗光电化学脱盐研究具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号