当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mutation effects on charge transport through the p58c iron–sulfur protein
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0sc02245d Ruijie D. Teo 1, 2, 3, 4 , Agostino Migliore 1, 2, 3, 4 , David N. Beratan 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0sc02245d Ruijie D. Teo 1, 2, 3, 4 , Agostino Migliore 1, 2, 3, 4 , David N. Beratan 1, 2, 3, 4, 5
Affiliation
|
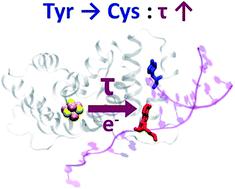
Growing experimental evidence indicates that iron–sulfur proteins play key roles in DNA repair and replication. In particular, charge transport between [Fe4S4] clusters, mediated by proteins and DNA, may convey signals to coordinate enzyme action. Human primase is a well studied [Fe4S4] protein, and its p58c domain (which contains an [Fe4S4] cluster) plays a role in the initiation of DNA replication. The Y345C mutation in p58c is linked to gastric tumors and may influence the protein-mediated charge transport. The complexity of protein–DNA systems, and the intricate electronic structure of [Fe4S4] clusters, have impeded progress into understanding functional charge transport in these systems. In this study, we built force fields to describe the high potential [Fe4S4] cluster in both oxidation states. The parameterization is compatible with AMBER force fields and enabled well-balanced molecular dynamics simulations of the p58c–RNA/DNA complex relevant to the initiation of DNA replication. Using the molecular mechanics Poisson–Boltzmann and surface area solvation method on the molecular dynamics trajectories, we find that the p58c mutation induces a modest change in the p58c–duplex binding free energy in agreement with recent experiments. Through kinetic modeling and analysis, we identify key features of the main charge transport pathways in p58c. In particular, we find that the Y345C mutation partially changes the composition and frequency of the most efficient (and potentially relevant to the biological function) charge transport pathways between the [Fe4S4] cluster and the duplex. Moreover, our approach sets the stage for a deeper understanding of functional charge transfer in [Fe4S4] protein–DNA complexes.
中文翻译:

突变对通过p58c铁硫蛋白的电荷传输的影响
越来越多的实验证据表明,铁硫蛋白在DNA修复和复制中起着关键作用。特别地,由蛋白质和DNA介导的[Fe 4 S 4 ]簇之间的电荷传输可能传递信号以协调酶的作用。人primase是一种经过充分研究的[Fe 4 S 4 ]蛋白,其p58c域(包含[Fe 4 S 4 ]簇)在DNA复制的起始过程中起作用。p58c中的Y345C突变与胃肿瘤有关,并可能影响蛋白质介导的电荷转运。蛋白质-DNA系统的复杂性以及[Fe 4 S 4的复杂电子结构团簇阻碍了对这些系统中功能电荷传输的理解。在这项研究中,我们建立了力场来描述高电势[Fe 4 S 4在两个氧化态下都成簇。该参数化与AMBER力场兼容,并实现了与DNA复制起始相关的p58c-RNA / DNA复合物的良好平衡的分子动力学模拟。在分子动力学轨迹上使用分子力学的泊松-玻尔兹曼和表面积溶剂化方法,我们发现p58c突变诱导了p58c-双链体结合自由能的适度变化,这与最近的实验一致。通过动力学建模和分析,我们确定了p58c主要电荷传输途径的关键特征。特别是,我们发现Y345C突变部分改变了[Fe 4 S 4之间]最有效(且可能与生物学功能相关)的电荷传输途径的组成和频率]群集和双工。此外,我们的方法为深入了解[Fe 4 S 4 ]蛋白质-DNA复合物中的功能性电荷转移奠定了基础。
更新日期:2020-07-15
中文翻译:

突变对通过p58c铁硫蛋白的电荷传输的影响
越来越多的实验证据表明,铁硫蛋白在DNA修复和复制中起着关键作用。特别地,由蛋白质和DNA介导的[Fe 4 S 4 ]簇之间的电荷传输可能传递信号以协调酶的作用。人primase是一种经过充分研究的[Fe 4 S 4 ]蛋白,其p58c域(包含[Fe 4 S 4 ]簇)在DNA复制的起始过程中起作用。p58c中的Y345C突变与胃肿瘤有关,并可能影响蛋白质介导的电荷转运。蛋白质-DNA系统的复杂性以及[Fe 4 S 4的复杂电子结构团簇阻碍了对这些系统中功能电荷传输的理解。在这项研究中,我们建立了力场来描述高电势[Fe 4 S 4在两个氧化态下都成簇。该参数化与AMBER力场兼容,并实现了与DNA复制起始相关的p58c-RNA / DNA复合物的良好平衡的分子动力学模拟。在分子动力学轨迹上使用分子力学的泊松-玻尔兹曼和表面积溶剂化方法,我们发现p58c突变诱导了p58c-双链体结合自由能的适度变化,这与最近的实验一致。通过动力学建模和分析,我们确定了p58c主要电荷传输途径的关键特征。特别是,我们发现Y345C突变部分改变了[Fe 4 S 4之间]最有效(且可能与生物学功能相关)的电荷传输途径的组成和频率]群集和双工。此外,我们的方法为深入了解[Fe 4 S 4 ]蛋白质-DNA复合物中的功能性电荷转移奠定了基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号