当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, development, testing at ISO standards and in vivo feasibility study of a novel polymeric heart valve prosthesis.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0bm00412j Joanna R Stasiak 1 , Marta Serrani , Eugenia Biral , James V Taylor , Azfar G Zaman , Samantha Jones , Thomas Ness , Francesco De Gaetano , Maria Laura Costantino , Vito D Bruno , Saadeh Suleiman , Raimondo Ascione , Geoff D Moggridge
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-06-17 , DOI: 10.1039/d0bm00412j Joanna R Stasiak 1 , Marta Serrani , Eugenia Biral , James V Taylor , Azfar G Zaman , Samantha Jones , Thomas Ness , Francesco De Gaetano , Maria Laura Costantino , Vito D Bruno , Saadeh Suleiman , Raimondo Ascione , Geoff D Moggridge
Affiliation
|
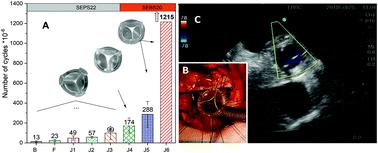
Clinically available prosthetic heart valves are life-saving, but imperfect: mechanical valves requiring anticoagulation therapy, whilst bioprosthetic valves have limited durability. Polymer valves offer the prospect of good durability without the need for anticoagulation. We report the design and development of a polymeric heart valve, its bench-testing at ISO standards, and preliminary extra-vivo and in vivo short-term feasibility. Prototypes were manufactured by injection moulding of styrenic block copolymers to achieve anisotropic mechanical properties. Design was by finite element stress–strain modelling, which has been reported previously, combined with feedback from bench and surgery-based testing using various combinations of materials, valve geometry and processing conditions. Bench testing was according to ISO 5840:2015 standards using an in vitro cardiovascular hydrodynamic testing system and an accelerated fatigue tester. Bench comparisons were made with a best-in-class bio-prosthesis. Preliminary clinical feasibility evaluations included extra-vivo and short-term (1–24 hours) in vivo testing in a sheep model. The optimised final prototype met the requirements of ISO standards with hydrodynamic performance equivalent to the best-in-class bioprosthesis. Bench durability of greater than 1.2 billion cycles (30 years equivalent) was achieved (still ongoing). Extra-vivo sequential testing (n = 8) allowed refinement of external diameter, 3D shape, a low profile, flexibility, suturability, and testing of compatibility to magnetic resonance imaging and clinical sterilisation. In vivo short-term (1–24 hours) feasibility (n = 3) confirmed good suturability, no mechanical failure, no trans-valvular regurgitation, competitive trans-valvular gradients, and good biocompatibility at histopathology. We have developed and tested at ISO standards a novel prosthetic heart valve featuring competitive bench-based hydrodynamics and durability, well beyond the ISO requirements and comparable to a best-in-class bioprosthesis. In vivo short-term feasibility testing confirmed preliminary safety, functionality and biocompatibility, supporting progression to a long-term efficacy trial.
中文翻译:

按照ISO标准进行设计,开发,测试以及新型聚合物心脏瓣膜假体的体内可行性研究。
临床上可使用的人工心脏瓣膜可挽救生命,但并不完美:机械瓣膜需要抗凝治疗,而生物人工瓣膜的耐用性有限。聚合物阀无需抗凝即可提供良好的耐用性。我们报告了聚合物心脏瓣膜的设计和开发,按照ISO标准进行的台架试验以及体外和体内的初步试验短期可行性。原型是通过注塑成型苯乙烯嵌段共聚物来实现各向异性的机械性能。设计是通过有限元应力应变模型进行的,该模型先前已有报道,并结合了使用材料,阀门几何形状和加工条件的各种组合进行的基于台式和基于手术的测试的反馈。基准测试是根据ISO 5840:2015标准,使用体外心血管水动力测试系统和加速疲劳测试仪进行的。使用同类最佳的生物假体进行基准比较。初步的临床可行性评估包括体内和短期(1-24小时)体内在绵羊模型中进行测试。经过优化的最终原型可以满足ISO标准的要求,其流体力学性能相当于同类最佳的生物假体。达到了超过12亿次循环(相当于30年)的工作台耐久性(仍在进行中)。体外顺序测试(n = 8)可以改善外径,3D形状,低轮廓,柔韧性,可保存性以及与磁共振成像和临床灭菌的相容性测试。体内短期(1–24小时)可行性(n= 3)确认具有良好的可缝合性,无机械故障,无跨瓣膜反流,竞争性跨瓣膜梯度和组织病理学上良好的生物相容性。我们已经按照ISO标准开发并测试了一种新型人工心脏瓣膜,该瓣膜具有基于台式的竞争性流体动力学特性和耐用性,远远超出了ISO要求,堪与同类最佳的生物假体相媲美。体内短期可行性测试证实了初步的安全性,功能性和生物相容性,为进行长期疗效试验提供了支持。
更新日期:2020-08-11
中文翻译:

按照ISO标准进行设计,开发,测试以及新型聚合物心脏瓣膜假体的体内可行性研究。
临床上可使用的人工心脏瓣膜可挽救生命,但并不完美:机械瓣膜需要抗凝治疗,而生物人工瓣膜的耐用性有限。聚合物阀无需抗凝即可提供良好的耐用性。我们报告了聚合物心脏瓣膜的设计和开发,按照ISO标准进行的台架试验以及体外和体内的初步试验短期可行性。原型是通过注塑成型苯乙烯嵌段共聚物来实现各向异性的机械性能。设计是通过有限元应力应变模型进行的,该模型先前已有报道,并结合了使用材料,阀门几何形状和加工条件的各种组合进行的基于台式和基于手术的测试的反馈。基准测试是根据ISO 5840:2015标准,使用体外心血管水动力测试系统和加速疲劳测试仪进行的。使用同类最佳的生物假体进行基准比较。初步的临床可行性评估包括体内和短期(1-24小时)体内在绵羊模型中进行测试。经过优化的最终原型可以满足ISO标准的要求,其流体力学性能相当于同类最佳的生物假体。达到了超过12亿次循环(相当于30年)的工作台耐久性(仍在进行中)。体外顺序测试(n = 8)可以改善外径,3D形状,低轮廓,柔韧性,可保存性以及与磁共振成像和临床灭菌的相容性测试。体内短期(1–24小时)可行性(n= 3)确认具有良好的可缝合性,无机械故障,无跨瓣膜反流,竞争性跨瓣膜梯度和组织病理学上良好的生物相容性。我们已经按照ISO标准开发并测试了一种新型人工心脏瓣膜,该瓣膜具有基于台式的竞争性流体动力学特性和耐用性,远远超出了ISO要求,堪与同类最佳的生物假体相媲美。体内短期可行性测试证实了初步的安全性,功能性和生物相容性,为进行长期疗效试验提供了支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号