当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrooxidation of C4 Polyols on Platinum Single-Crystals: A Computational and Electrochemical Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jpcc.0c05017 Gabriela Soffiati 1 , José L. Bott-Neto 1, 2 , Victor Y. Yukuhiro 1, 2 , Cléo T. G. V. M. T. Pires 1 , Carlos C. Lima 1 , Cinthia R. Zanata 3 , Yuvraj Y. Birdja 4 , Marc T. M. Koper 4 , Miguel A. San-Miguel 1 , Pablo S. Fernández 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-06-16 , DOI: 10.1021/acs.jpcc.0c05017 Gabriela Soffiati 1 , José L. Bott-Neto 1, 2 , Victor Y. Yukuhiro 1, 2 , Cléo T. G. V. M. T. Pires 1 , Carlos C. Lima 1 , Cinthia R. Zanata 3 , Yuvraj Y. Birdja 4 , Marc T. M. Koper 4 , Miguel A. San-Miguel 1 , Pablo S. Fernández 1, 2
Affiliation
|
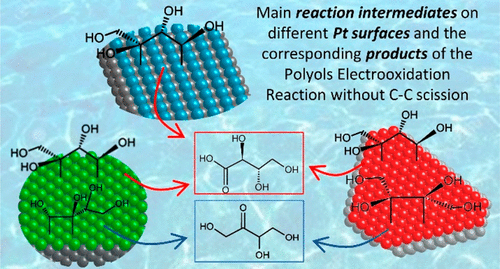
Many polyols are abundant and cheap molecules highly spread in the biomass. These molecules have an enormous potential to be used in electrochemical devices to generate energy and/or value-added molecules. The electrooxidation of polyols can produce different substances of interest in the chemical industry concomitantly to high purity hydrogen in electrolyzers. The cost in the production of all these chemicals depends, among other factors, on the develop of more active and selective catalysts. However, in order to search for these materials using computational experiments, it is mandatory to have a better understanding of the fundamental aspect of the reactions, which permit to base the search on the adsorption energies of one or more key reaction intermediates. To contribute to this task, we performed (spectro)electrochemical and computational experiments to study the electrooxidation of C4 polyols. We show that the electrooxidation of polyols does not depend on the relative orientation of their OH groups. Besides, using Pt single crystals, we demonstrate that the trend for the oxidation of the primary carbon (relative to the secondary) increases in the order Pt(111) < Pt(100) < Pt(110) and that this result can be extended to polyols with longer carbon chains. Finally, computational experiments permit us to rationalize these trends looking at the relative stability of double dehydrogenated intermediates on the Pt basal planes.
中文翻译:

铂单晶上C 4多元醇的电氧化:计算和电化学研究
许多多元醇丰富且廉价,分子高度分散在生物质中。这些分子具有用于电化学装置中以产生能量和/或增值分子的巨大潜力。多元醇的电氧化可在化学工业中产生与电解槽中高纯度氢同时产生的不同的目标物质。除其他因素外,所有这些化学品的生产成本取决于开发更具活性和选择性的催化剂。但是,为了使用计算实验来搜索这些材料,必须对反应的基本方面有更好的了解,这可以使搜索基于一种或多种关键反应中间体的吸附能。为了完成这项任务,4种多元醇。我们表明,多元醇的电氧化并不取决于其OH基团的相对取向。此外,使用Pt单晶,我们证明伯碳的氧化趋势(相对于仲碳)以Pt(111)<Pt(100)<Pt(110)的顺序增加,并且可以扩展该结果碳链较长的多元醇。最后,计算实验使我们能够合理地研究这些趋势,着眼于Pt基面上的双脱氢中间体的相对稳定性。
更新日期:2020-07-09
中文翻译:

铂单晶上C 4多元醇的电氧化:计算和电化学研究
许多多元醇丰富且廉价,分子高度分散在生物质中。这些分子具有用于电化学装置中以产生能量和/或增值分子的巨大潜力。多元醇的电氧化可在化学工业中产生与电解槽中高纯度氢同时产生的不同的目标物质。除其他因素外,所有这些化学品的生产成本取决于开发更具活性和选择性的催化剂。但是,为了使用计算实验来搜索这些材料,必须对反应的基本方面有更好的了解,这可以使搜索基于一种或多种关键反应中间体的吸附能。为了完成这项任务,4种多元醇。我们表明,多元醇的电氧化并不取决于其OH基团的相对取向。此外,使用Pt单晶,我们证明伯碳的氧化趋势(相对于仲碳)以Pt(111)<Pt(100)<Pt(110)的顺序增加,并且可以扩展该结果碳链较长的多元醇。最后,计算实验使我们能够合理地研究这些趋势,着眼于Pt基面上的双脱氢中间体的相对稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号